2021 Medical Reports Summarize a Cardiovascular, Thromboembolic (clotting) Safety Disaster due to the mRNA technology gene based vaccines & McCullough is sage as he waxes scholarly on the
catastrophe; Let us face it, the Pfizer, Moderna, BioNTech mRNA vaccines (& DNA platforms even NOVOVAX); Systematic Review of Published Serious COVID-19 Vaccine Adverse Events (YASMIN et al.)
https://pubmed.ncbi.nlm.nih.gov/36988252/
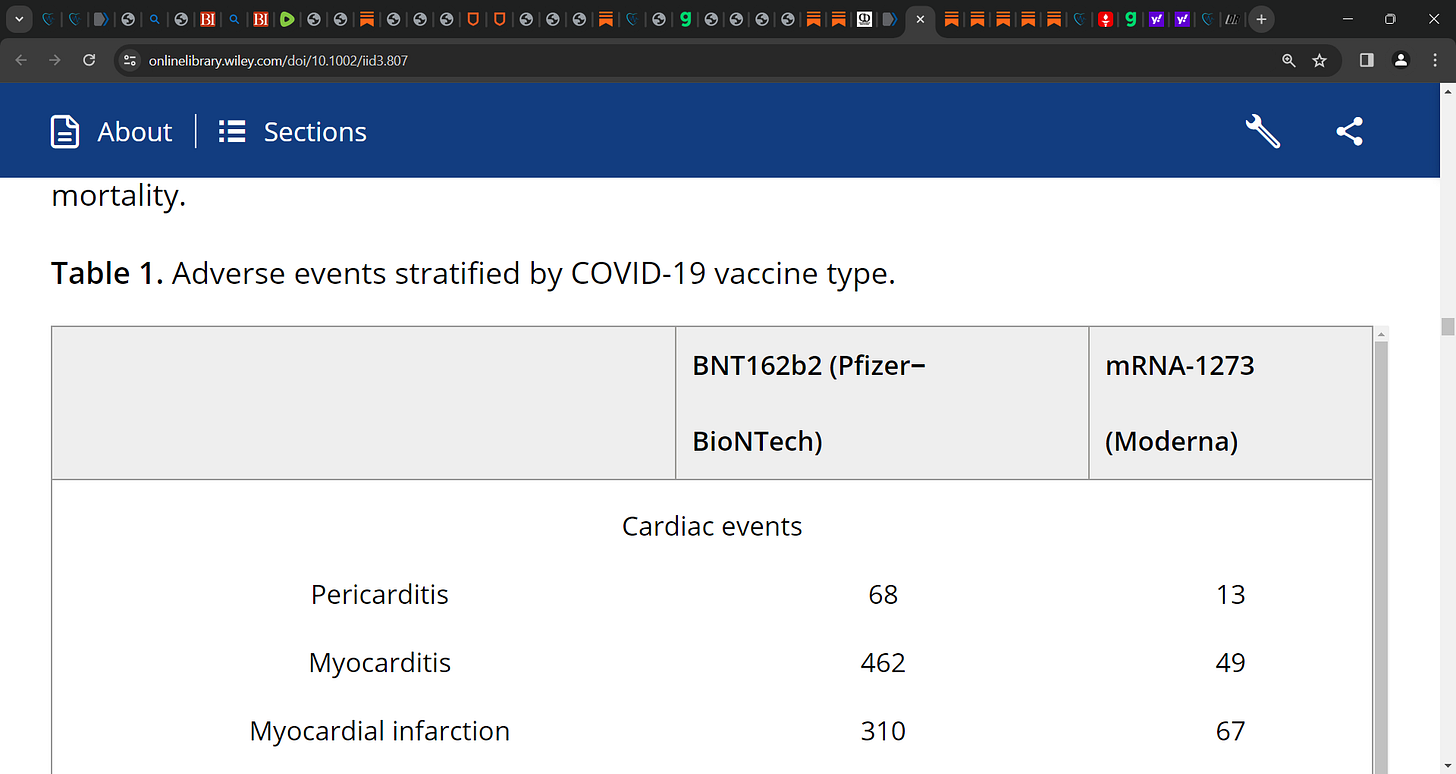
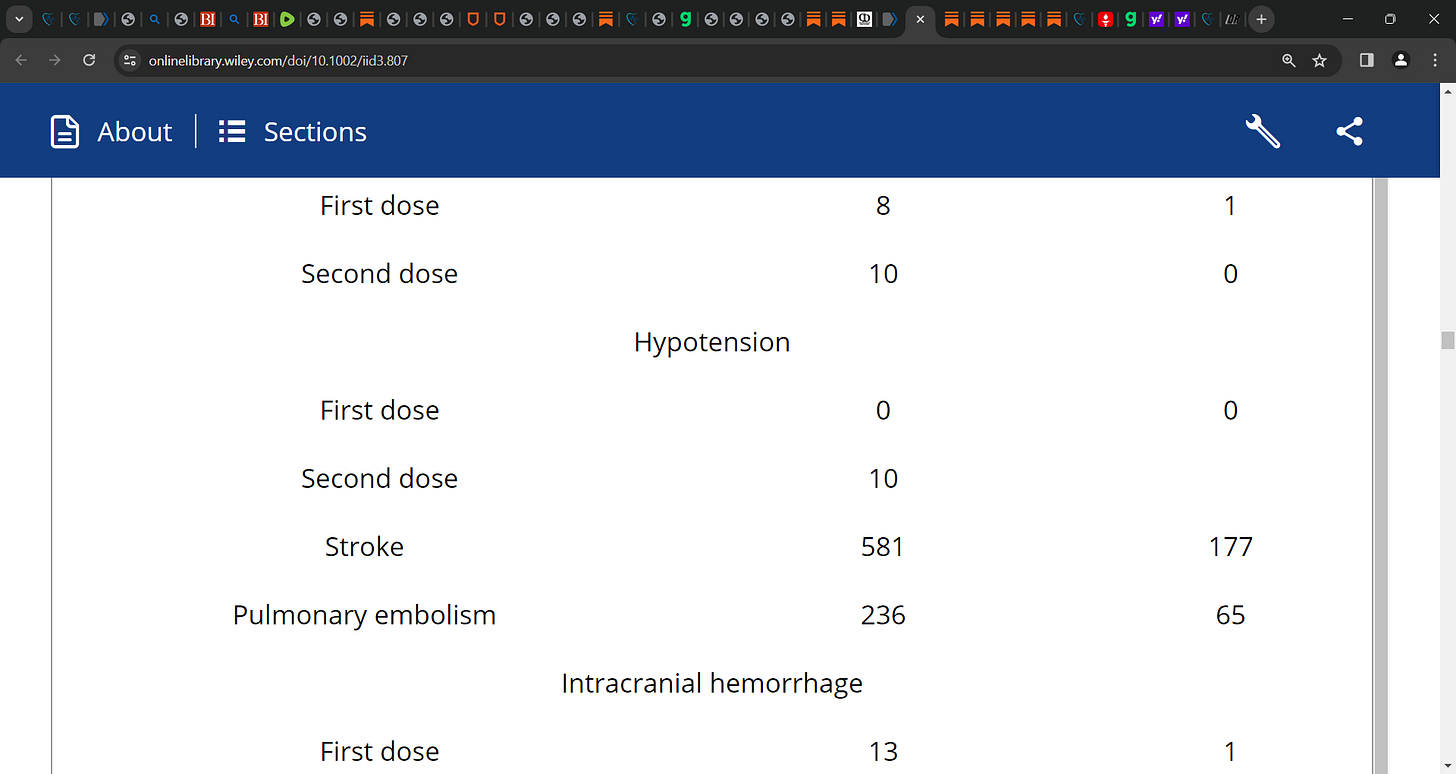
There are various cardiovascular adverse events reported after the mRNA vaccines' first or second dose including pericarditis/myopericarditis, myocarditis, hypotension, hypertension, arrhythmia, cardiogenic shock, stroke, myocardial infarction/STEMI, intracranial hemorrhage, thrombosis (deep vein thrombosis, cerebral venous thrombosis, arterial or venous thrombotic events, portal vein thrombosis, coronary thrombosis, microvascular small bowel thrombosis), and pulmonary embolism.
‘Methods: A systematic review of original studies reporting confirmed cardiovascular manifestations post-mRNA COVID-19 vaccination was performed…
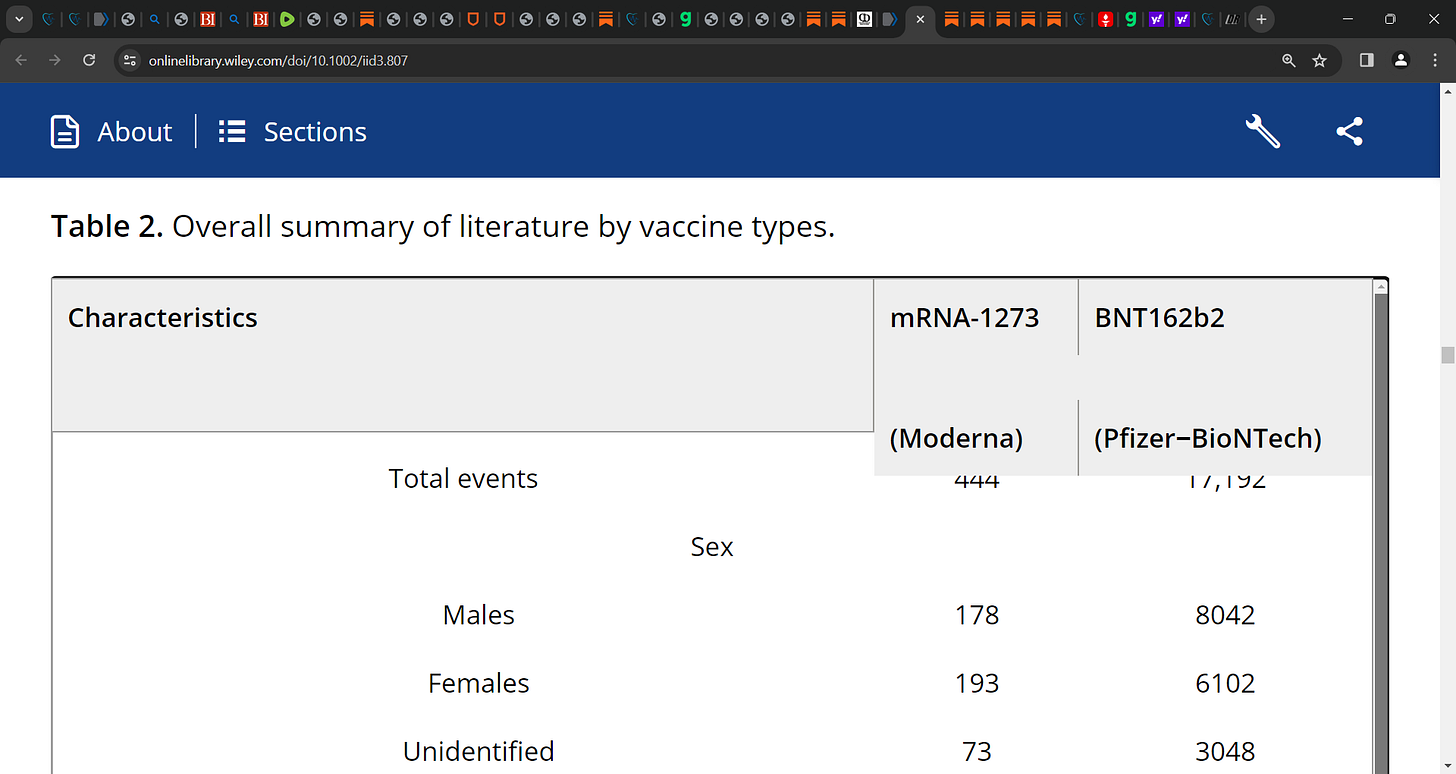
Results: A total of 81 articles analyzed confirmed cardiovascular complications post-COVID-19 mRNA vaccines in 17,636 individuals and reported 284 deaths with any mRNA vaccine.
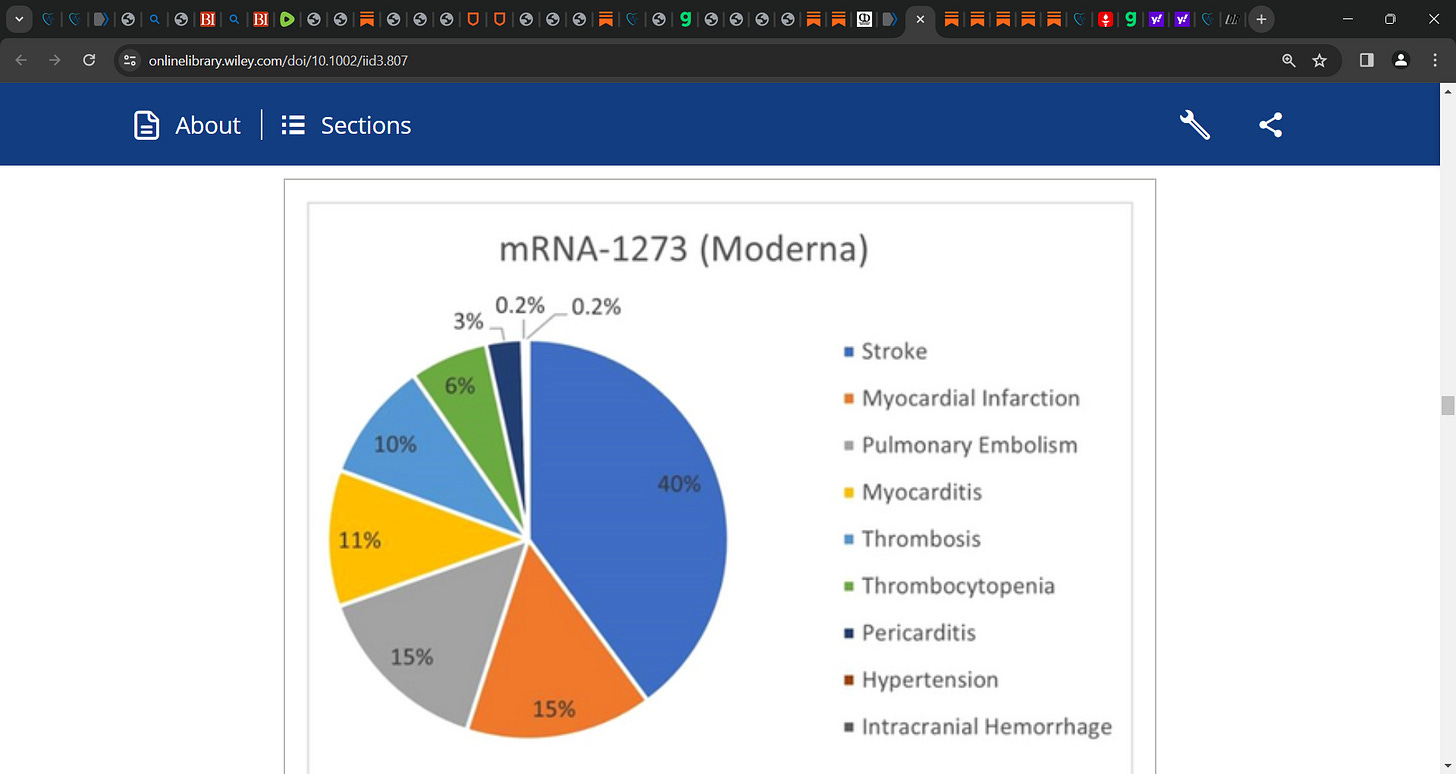
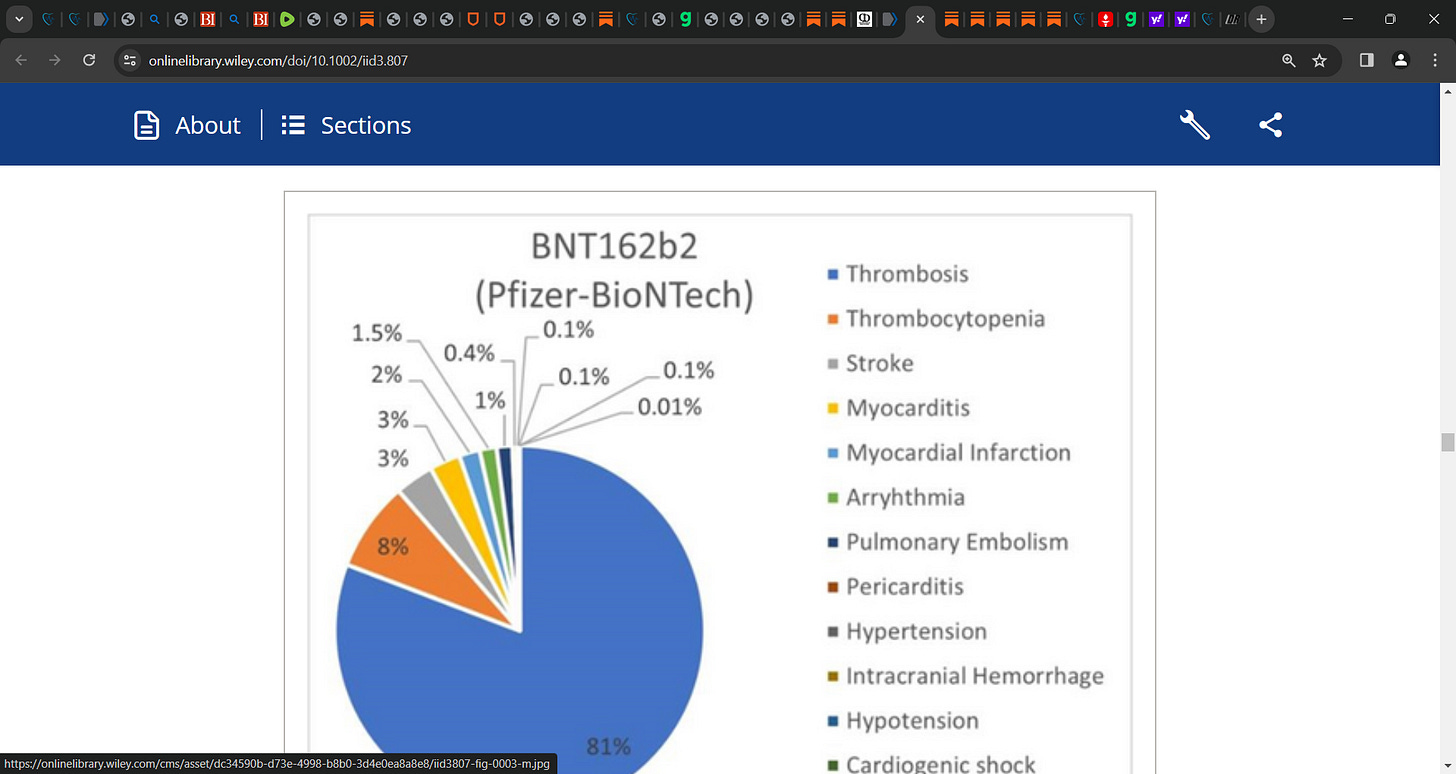
Of 17,636 cardiovascular events with any mRNA vaccine, 17,192 were observed with the BNT162b2 (Pfizer-BioNTech) vaccine, 444 events with mRNA-1273 (Moderna).
Thrombosis was frequently reported with any mRNA vaccine (n = 13,936), followed by stroke (n = 758), myocarditis (n = 511), myocardial infarction (n = 377), pulmonary embolism (n = 301), and arrhythmia (n = 254).
Stratifying the results by vaccine type showed that thrombosis (80.8%) was common in the Pfizer BNT162b2 cohort, while stroke (39.9%) was common with mRNA-1273 for any dose.
The time between the vaccination dosage and the first symptom onset averaged 5.6 and 4.8 days with the mRNA-1273 vaccine and BNT162b2, respectively. The mRNA-1273 cohort reported 56 deaths compared to the 228 with BNT162b2, while the rest were discharged or transferred to the ICU.’
The average time between the vaccination dosage and the onset of the first symptom was 5.6 days. Individuals requiring hospitalization had a median length of 3.7 days of hospital stay. The study synthesizes data of abnormal or elevated lab values; 13 events were reported for elevated CRP levels, 5 individuals had increased Troponin T; whereas 8 cases observed an increase in Troponin I levels.
ECG and cardiac magnetic resonance imaging were the commonly used diagnostic procedures among hospitalized patients for any adverse outcomes. Data reveals 16 cases of ST-elevations and 7 PR-depressions, followed by ST-depressions, T-wave abnormalities, and bundle branch blocks.
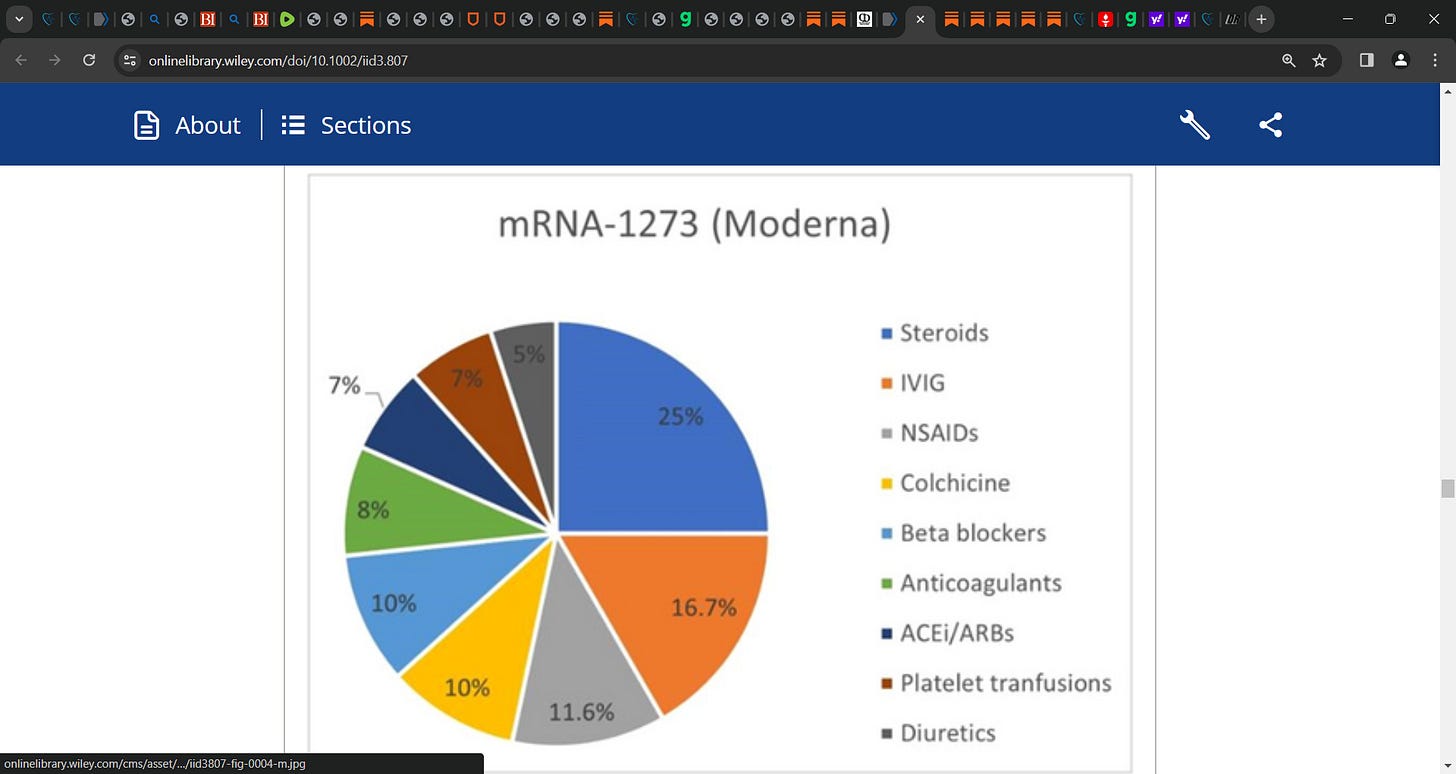
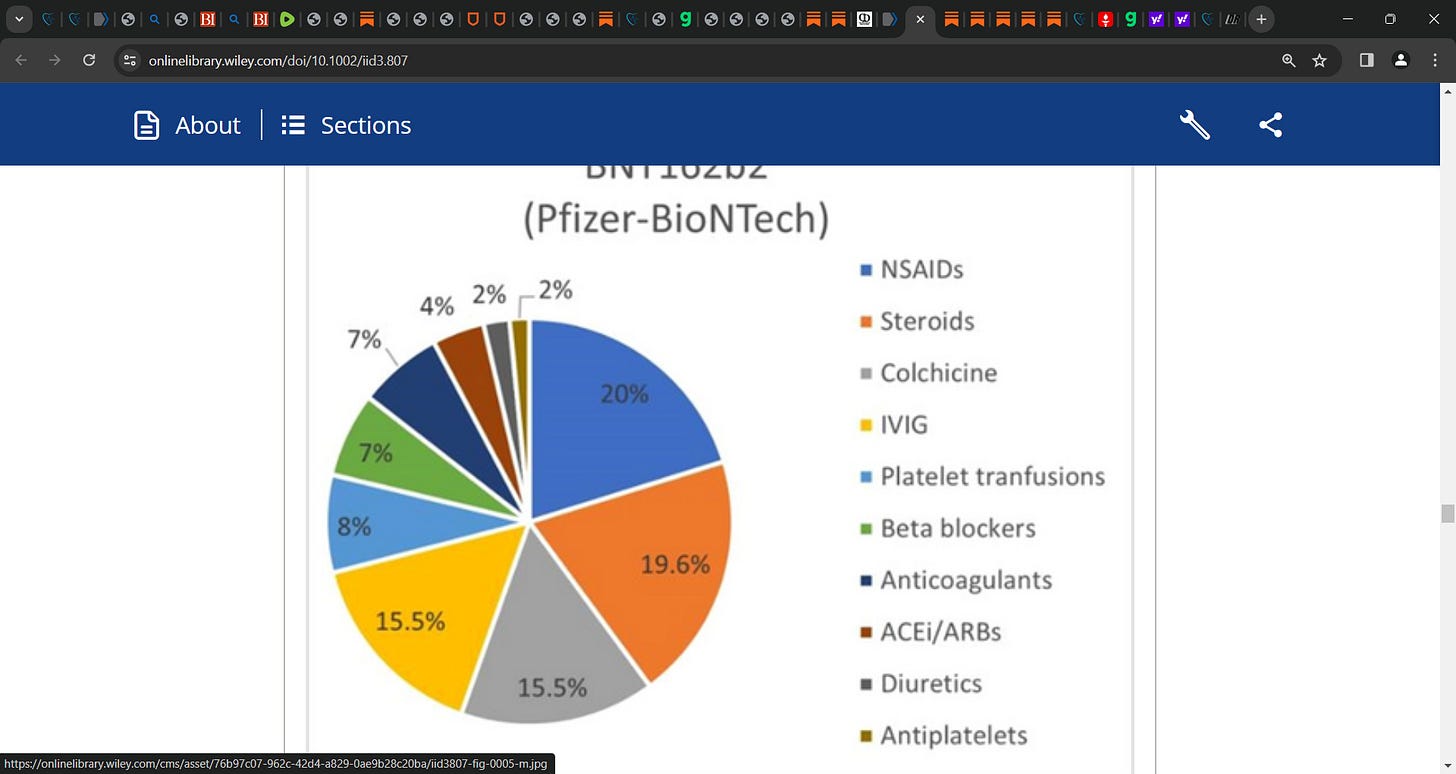
In studies that reported treatment options steroids (n = 15 cases) including corticosteroids, prednisone, methylprednisolone, and dexamethasone frequently opted for. This was followed by IVIG in 10 cases, NSAIDs in 7, and colchicine in 6 cases, respectively. Analysis of literature reported mortality in 56 cases (12.6%); with the rest being discharged or transferred to ICU.
‘4 DISCUSSION
This systematic review evaluated the rare CV complications that have occurred due to mRNA vaccines and revealed that the highest complication reported combined for both mRNA vaccines was thrombosis. Thrombocytopenia has also been widely recorded and was the second-highest adverse event to occur. Our analysis also demonstrated a moderate frequency of vascular adverse events among which, stroke was most reported and was overall, the third-highest event to be documented. Myocarditis was the next commonly occurring complication and had the highest cases reported among all cardiac events. Other cardiac events included MI, arrhythmia, pericarditis, and cardiogenic shock. Cardiogenic shock was the lowest reported adverse effect among all events.
The mRNA vaccines have been graded as the most effective (approximately 94%) due to their strong immunogenicity and effective presentation of SARS-CoV-2 antigens to the immune system. Real-world incidence of adverse reactions after mRNA vaccines has also remained lower than that concluded from clinical trials. Several systematic reviews have been published since the onset of the SARS-CoV-2 infection evaluating the relation between CV conditions and COVID-19 outcomes. Cardiac complications have been observed to be the most frequently occurring complication while CV disease is the most common comorbidity.15 A few studies have explored the incidence of thrombosis, thrombocytopenia, and various vascular events. However, the evolving situation with the emergence of new SARS-CoV-2 variants requires that the focus of healthcare experts be on investigating the course of any adverse event reported concerning the vaccination phase. We presume that a thorough analysis of all adverse events arising with mRNA vaccination is necessary to know the details of these events and adopt measures to mitigate the difficulties accordingly. Recent studies analyzing complications occurring post-mRNA vaccination have focused on a single or few complications while our study has extensively reviewed literature published so far and has considered all potential studies. For the short-term knowledge regarding adverse effects of mRNA vaccination,16 our findings provide a comprehensive insight into the unfavorable sequel that has been reported and stratify them based on the different types of mRNA vaccines. This can be possibly helpful in better understanding and predicting the intricacies of mRNA vaccine inoculation.’
See McCullough’s strong work and support him.