FDA & CDC will cover this up!: "Acquired hemophilia A following COVID-19 vaccination, The importance of prompt diagnosis: A case report of cutaneous hematoma occurring 8 days after COVID vaccination."
See my bolded text & abstract of this breaking study report; the FDA and CDC and NIH and Fauci and Ashish will pretend this does not exist, & will smear me and McCullough & Risch for highlighting it
Abstract
‘Acquired hemophilia A (AHA) is a rare coagulopathy characterized by hemorrhagic manifestations. It has been linked to various conditions, including autoimmune disorders, drugs, tumors, lymphoproliferative disorders, and infections.
We present a case of AHA in a 71-year-old male patient with cutaneous hematoma occurring 8 days after vaccination for COVID-19. This report aims to highlight the risk of FVIII inhibitor development following an immune stimulus, thus improving our knowledge regarding possible vaccination-related adverse events.
Furthermore, we underline how the potential risk of not recognizing disease manifestations promptly, together with specific coagulation alterations, could significantly affect the patient's outcome. Adequate management plans and the diffusion of shared guidelines are of fundamental importance in order to prevent the development of life-threatening complications and initiate appropriate treatment as soon as possible.’
Case presentation
A 71-year-old male patient was admitted to Emergency Department (ED) in another center, reporting right arm pain and swelling associated with limited range of motion, together with a non-traumatic onset of diffuse petechiae, eight days after receiving the second dose of COVID-19 vaccine (BNT162b2, Comirnaty, Pfizer/BioNTech).
Laboratory exams showed prolonged activated partial thromboplastin time (APTT) – 67.6 s (normal range 25.1–38 s). No alteration in coagulation studies had ever been reported in the patient's past medical history. Doppler ultrasound of the superficial and deep vessels of the right arm was negative for venous thrombosis. No evidence of soft tissue hematomas was found. The patient was later discharged with outpatient therapy based on antibiotics, anticoagulants (enoxaparin 4000 UI – despite the absence of thrombosis evidence and based only on clinical suspicion of the clinicians), and painkillers.
The following day, he was admitted to ED in our hospital due to the worsening clinical manifestation. Massive spontaneous hematomas were evidenced on the physical exam, including the left hemithorax and the abdomen, extending from the pelvic area to the right thigh (Fig. 1). These signs were accompanied by further swelling of the right arm and the onset of paresthesia. Complete blood count showed mild anemia (11.6 g/dL – normal range 13–16 g/dL), and normal platelet (237 ×109/L – normal range 150–500 ×109/L) count. The coagulation profile confirmed prolonged APTT (72 s). At the same time, the mixing study showed immediate correction at time 0, which was, however, prolonged once again after two hours of incubation at 37 °C (98,6°F), suspecting the presence of time- and temperature-dependent clotting factor inhibitors.
Acquired hemophilia onset was immediately suspected due to the possible presence of time-dependent clotting factor inhibitors and confirmed with low FVIII (7.9 %, normal value 70–130 %), associated with the presence of high FVIII autoantibody inhibitor titer (64 Bethesda Unit – BU). In past medical history, colorectal cancer was treated surgically fifteen years ago with right hemicolectomy and has been regularly monitored with colonoscopy at least once every 2 years since then. The patient also underwent multiple endoscopic polypectomies and cholecystectomy for gallstones. The patient had no history of bleeding, clotting, or other autoimmune disorders. To exclude possible secondary organic causes of hemophilia A, CT-total body was performed with no signs of malignancy.
Screening for autoimmune (anti-nuclear antibody, anti-extractable nuclear antigen, anti-double-stranded DNA), lupus anticoagulant studies, and chronic infectious diseases also resulted negative. Colonoscopy was also performed in order to exclude recurrent colorectal cancer and confirmed multiple polyps, with no signs of neoplastic nature in a few of them that were biopsied. In molecular biology, screening for viral infections (HBV, HCV, HIV, CMV, EBV) tested negative, while blood, urine, and stool cultures excluded other underlying infectious conditions. Serum immunofixation and free light chain study excluded the presence of monoclonal protein. Considering the absence of well-identified secondary causes that could have triggered the development of FVIII antibodies and the close temporal correlation with the COVID-19 vaccine, we hypothesized AHA onset following immune vaccine stimulation.
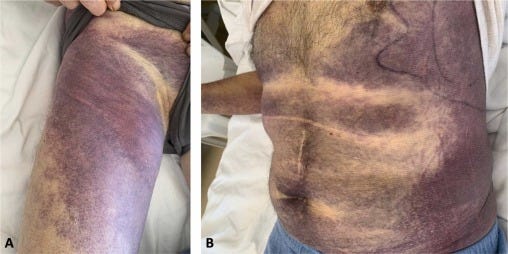
So, during the hospitalization, in a couple of days, the hemoglobin value continued to decrease (6.7 g/dL) in concomitance of hematoma enlargement, so the patient received transfusion support with red blood cell units (3 total units in a week). The patient was immediately started on anti-hemorrhagic treatment with recombinant activated factor VII - rFVIIa - bypassing agent (90 mg/kg every 3 h during active bleeding), associated with immunosuppressive prednisone therapy (1 mg/kg), in order to eradicate the FVIII inhibitors. Despite the rFVIIa administration, the patient showed a slight improvement in the following days, with a further Hb value drop (6.4 g/dL) in concomitance of subcutaneous hematoma enlargement and persistent low FVIII activity. Therefore, the patient received transfusion support with red blood cell units (3 total units per week). Cyclophosphamide 2 mg/kg daily was added to prednisone as a combination of immunosuppressive therapy. Seven days following hospital admission, the patient showed clinical improvement with hematoma resolution, increased hemoglobin value, and a progressive rise in FVIII activity (Fig. 2). Later, anti-hemorrhagic therapy with rFVIIa was subsequently reduced gradually in fixed intervals every 6–8 h and every 12 h until treatment suspension.
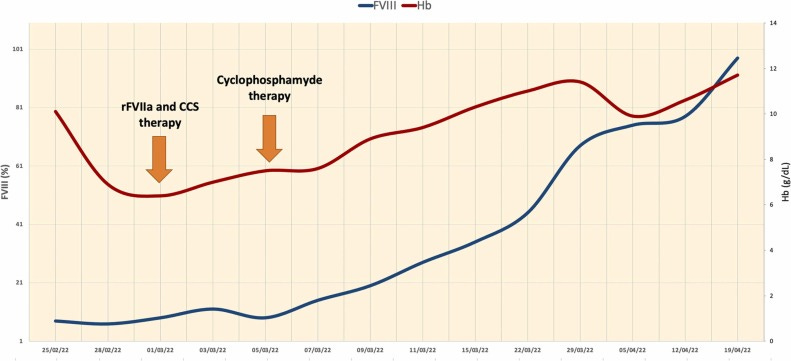
The patient was discharged ten days after the start of cyclophosphamide therapy with stable both Hb value (9.2 g/dL) and FVIII activity (92 %), normal APTT (39 s), and no signs or symptoms of bleeding, continuing steroid, and oral cyclophosphamide therapy, and regular hematological follow-up. After immunosuppressive tapering and later treatment discontinuation, the patient remains in response with no signs or symptoms of new bleeding, normal values of both FVIII and APTT values at the last follow-up, while autoantibody levels remain low.
3. Discussion
Anti-FVIII autoantibodies inhibit the regular functional activity of endogenous FVIII in AHA, leading to a hemorrhagic diathesis. Factor VIII, together with activated factor IX (FIXa) and the simultaneous release of Ca2+ and phospholipids, forms a complex that activates factor X (FXa), allowing blood clot formation. Clinically, the primary manifestation of AHA is bleeding. It is suspected in individuals who report acute or recurrent bleeding symptoms, with no previous history of bleeding episodes, associated with prolonged isolated APTT. In the absence of other clinical explanations (e.g., pharmacological anticoagulants), APTT mixing study (usually prolonged) and factor VIII activity are necessary diagnostic tools. The diagnosis of AHA is eventually confirmed by the finding of autoantibodies, using the Bethesda assay or with enzyme-linked immunosorbent assay (ELISA) [[5]]. Prompt timeliness and organized diagnostic-therapeutic actions are crucial in newly diagnosed AHA management.
The therapeutic approach aims at three fundamental objectives: to quickly stop bleeding through plasma FVIII bypassing or concentrating agents and eliminate the risk of further bleeding, eradicate the inhibitor with adequate immunosuppressive therapy, and treat the underlying cause, when recognized [[6]].
The causes are often misunderstood, and possible events that trigger the autoimmune process, such as infectious episodes or drug use, generally involve immune system response leading to anti-FVIII production. The pathophysiology is unclear, although it is thought that T-lymphocytes and specific genetic polymorphisms may play a role [[7]]. It has also been suggested that vaccination may trigger an autoimmune response due to antigenic mimicry and activation of quiescent auto-reactive T and B cells, leading to autoantibody development [[8]].
Case reports describing AHA onset post-infection COVID-19, including asymptomatic SARS-CoV-2 infections, highlight their potential impact as associated autoimmune phenomena [[9]].
In the last years, with the development of the vaccines for SARS-CoV-2 and their wide use in the world population in a brief period, several cases of patients who have developed autoimmune disorders have been reported in the literature. Namely, both onset and relapse of immune thrombocytopenic purpura [[10]] and immune-mediated thrombotic thrombocytopenic purpura [[11]] were described in the literature, along with a new condition characterized by thrombocytopenia accompanied by thrombosis and antibodies against platelet factor 4 (PF4) in the serum (defined vaccine-induced thrombotic thrombocytopenic -VITT-) [[12]]. Finally, like in our patient, cases of AHA onset following vaccination have been described, thus improving our knowledge of the possible underlying pathophysiological mechanisms, although establishing a specific causal relationship between the vaccine and these autoimmune disorders can be challenging due to several variables related to a single case [13, 14, 15, 16]. Despite difficulties in establishing a specific correlation, several working groups, including Leone and colleagues, have reported an increase in the incidence of AHA in the months following the worldwide SARS-CoV-2 immunization period (between 14 and 52 days from vaccination day and AHA-related treatment start) [[17]].
In these cases, reactivation of quiescent B- and T-lymphocytes could represent a possible underlying due to the cross-reactivity of antibodies against SARS-CoV-2 spike glycoproteins to structurally similar molecules belonging to the coagulation factors. On the other hand, according to Hirsinger et al., the possible association of autoimmune disorders with COVID-19 mRNA vaccination was probably not due to anti-S-IgG anti-S-IgG cross-reacting FVIII inhibitors induced by the vaccine. Instead, polyclonal activation of B lymphocytes could lead to the production of autoantibodies in pre-existing self-reactive B-cell clones of individuals already predisposed to developing autoimmune diseases [[18]]. However, further studies are needed to confirm these hypotheses.
Regarding our case, it appears evident that the patient was not appropriately managed initially due to an unrecognized AHA diagnosis and the inappropriate and dangerous use of anticoagulant therapy in the absence of thrombosis on Doppler ultrasound. AHA represents a medical emergency, and given the clinical manifestations, it is frequently managed in an emergency room setting, where due to the physicians’ inexperience, it can lead to delays in diagnosis, aggravating the prognosis, and causing a waste of resources (e.g., useless diagnostic investigations, hold of adequate therapeutic strategies, days of hospitalization). According to the data reported by EACH2, the period between admission and definitive AHA diagnosis exceeds seven days in most cases [[19]].
Finally, it is fundamental to start therapy with a bypassing agent (e.g., either procoagulant recombinant activated factor or anti-inhibitor coagulant complex with similar results) as soon as possible as concurrent immunosuppressive drugs. If the steroid therapy is inadequate to eradicate the inhibitor, second or third lines of treatment with drugs such as cyclophosphamide or rituximab can play a pivotal role and improve patient outcomes, as in our case. In the cases of AHA following vaccination described in the literature, inhibitor levels are generally responsive to steroid therapy alone (e.g., 2 BU in 4 weeks, as described in the case of Radwi et al. [[16]]), avoiding the need for other immunosuppressive therapies. In our case, we performed another Bethesda assay to quantify the value before steroid withdrawal, two months after the start of treatment (<0.4 BU), confirming the early inhibitor eradication.
4. Conclusion
The onset of AHA is an often-misunderstood condition with delayed diagnosis, and pathogenetic mechanisms should be more defined. The SARS-CoV-2 pandemic and the extensive vaccination campaign implemented worldwide could be an important trigger mechanism for autoimmune manifestations, especially in predisposed individuals. Coagulation disorders such as AHA can be potentially lethal. Increasing awareness of this rare side-effect following vaccination and its surveillance could help our knowledge of the underlying mechanisms and improve patient outcomes.’
SOURCE:
https://www.trasci.com/article/S1473-0502(22)00269-5/fulltext



Page 33 of 38. Lots of Hemorrhage Disorders listed on Pfizer’s Clinical Trial documents. Just more evidence.
https://phmpt.org/wp-content/uploads/2021/11/5.3.6-postmarketing-experience.pdf
My aunt just received this dx?? She is 88 and lives in an assisted living facility- they assumed she fell out of bed. They are still giving her transfusions at the hospital. Her body looks like these pictures?? This is awful.