IGOR CHUDOV once again presents superb scholarship as he unpacks NEJM published data on the new mRNA vaccine for RSV immunization (mRNA-1345); is this another mRNA technology vaccine fraud?
Support Igor; asks rightly if you look at data, why would trial arm subjects (injected) get more pneumoia & serious effects? data shows this vaccine to be ineffective & harmful? FDA to approve?
Excellent scholarship, analysis by Igor!
I remain an admirer of his work!
Start here:
‘A new mRNA vaccine is being developed: Moderna’s mRNA-1345 is a proposed immunization against RSV, one of the viruses causing colds in adults. Clinical trial results were published in the New England Journal of Medicine today.
The clinical trial’s 35,541 participants were assigned to receive the mRNA-1345 vaccine (17,793 participants) or placebo (17,748). (The placebo was saline.)
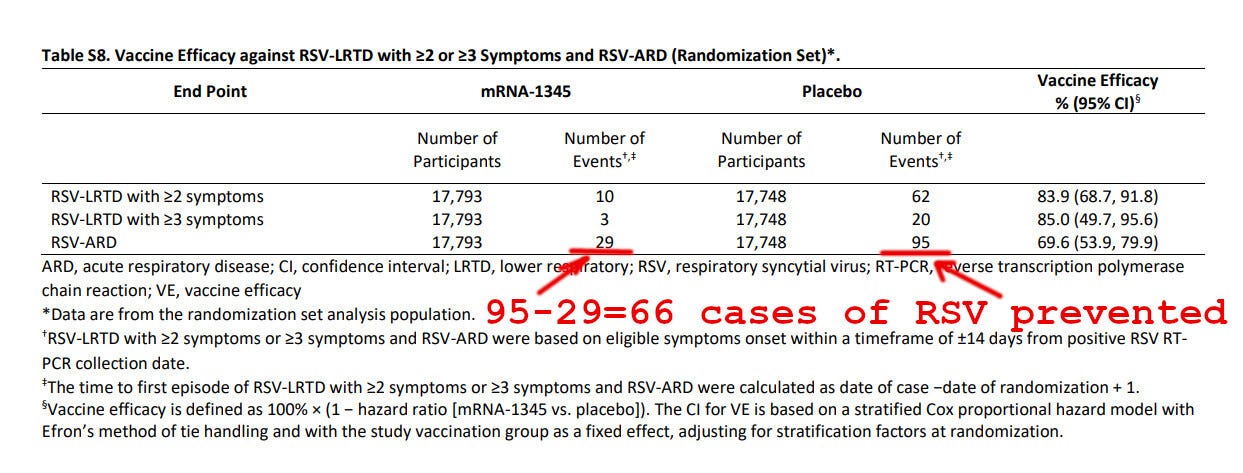
Moderna proudly reported that its RSV vaccine prevented 66 cases of RSV in 17 thousand vaccinated people. Per the appendix:
The Moderna study explains:
Vaccine efficacy was 68.4% (95% CI, 50.9 to 79.7) against RSV-associated acute respiratory disease. Protection was observed against both RSV subtypes (A and B) and was generally consistent across subgroups defined according to age and coexisting conditions.
mRNA RSV vaccine efficacy of just 68% was relatively meager, far worse than the much-ballyhooed “95% efficacy” of COVID vaccines.
The interesting part follows:
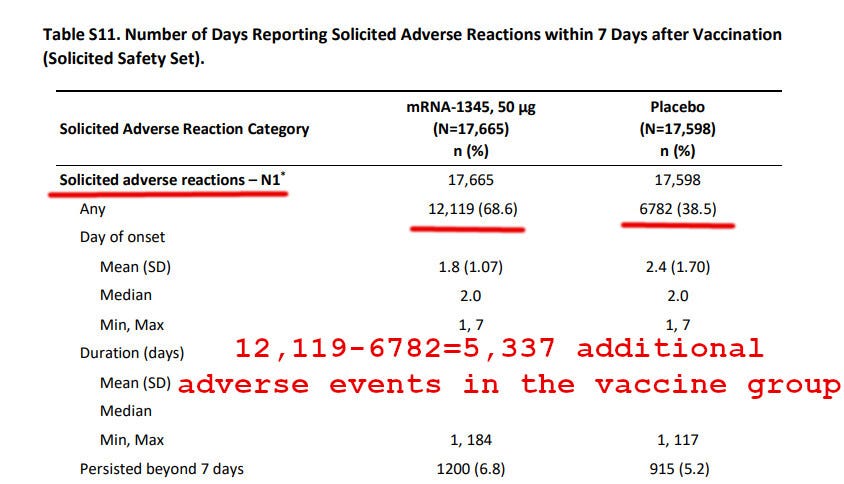
Participants in the mRNA-1345 group had a higher incidence than those in the placebo group of solicited local adverse reactions (58.7% vs. 16.2%) and of systemic adverse reactions (47.7% vs. 32.9%); most reactions were mild to moderate in severity and were transient.
Was taking the RSV vaccine worth it for the trial participants? Consider this: as per the above image, the vaccine prevented 66 cases of RSV among 17,793 vaccine recipients. However, it caused 5,337 more adverse events in the vaccine group than in the placebo group.
Adverse events were varied, ranging from trivial, such as minor headaches, to more disturbing ones, such as underarm lymph node swelling, fevers, vomiting, etc.
Overall, for each prevented case of RSV (remember, the vaccine prevented only 66 cases), the vaccinated subgroup suffered 5337/66 = 81 adverse events.
Let me repeat: there were 81 adverse events per each prevented case of RSV.
Is having 81 adverse events per case of RSV, essentially a mild cold, a good deal? Not to me!
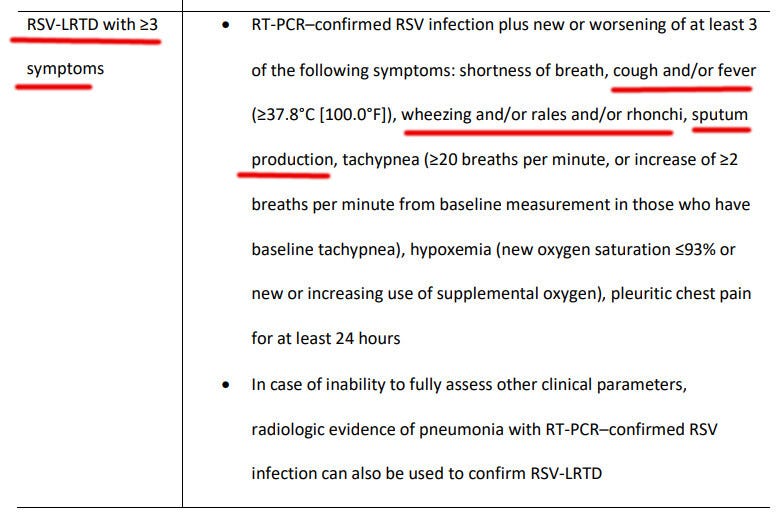
What about preventing more serious illness? As per the vaccine efficacy picture below, the RSV vaccine prevented 17 cases of RSV-LRTD with >= three symptoms.
Mind you, these were not hospitalizations — merely instances of RSV with more symptoms than usual.
For example, if the patient had a 1) cough, 2) fever over 100F, and 3) sputum production, it would fit the above-underlined definition:
How many adverse events would the RSV-vaccinated study subjects have to suffer to prevent 17 such instances? The math works out to 5,337 vaccine adverse events, preventing 17 cases of RSV-LRTD with >= 3 symptoms, or 5337/17 = 313 adverse events per prevented RSV illness with at least three symptoms.
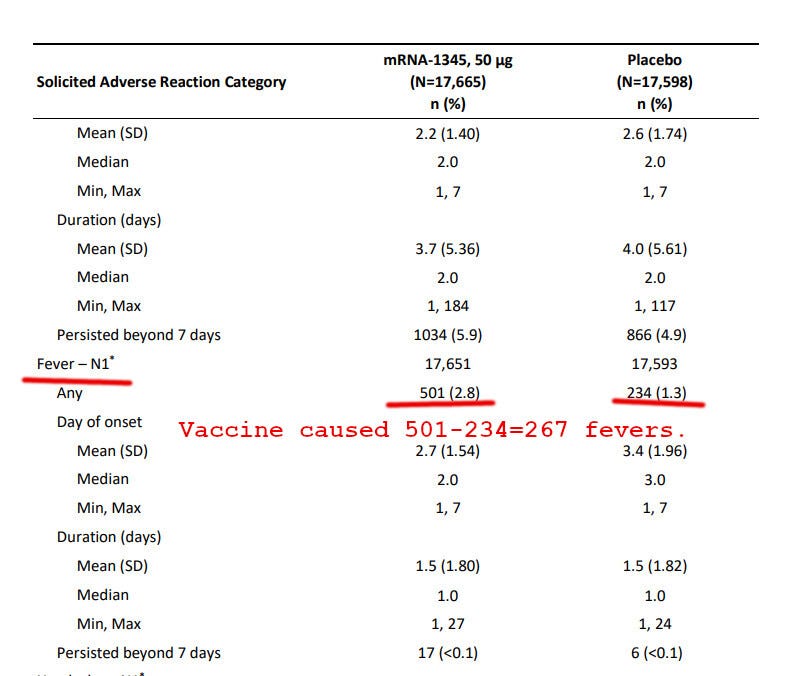
Did all 17 of the above instances involve fevers? Moderna’s study is silent on this, but let’s give them the benefit of the doubt and assume that all the above-mentioned prevented cases had a fever.
How many vaccinations resulted in vaccine-caused fevers? Page 53 helpfully provides information:
So, to prevent about 17 worse-than-usual cases of RSV involving fevers, the vaccinated subgroup had to suffer through 267 vaccine-caused fevers. Similarly, to prevent all 66 cases of RSV, avoided in the vaccinated subgroup, the vaccinees had to suffer through 267 fevers caused by the RSV vaccine.
Is that a good risk-benefit tradeoff?
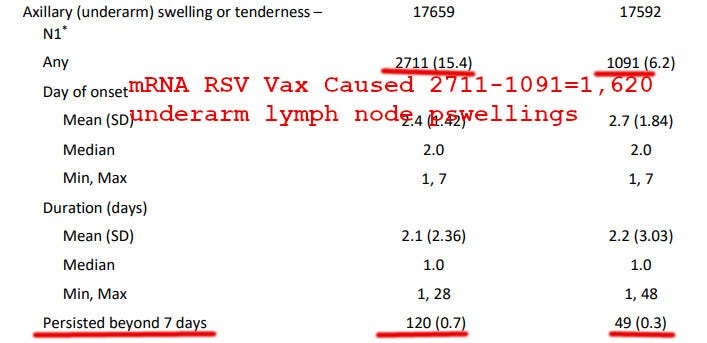
Underarm Swelling
More worryingly, the mRNA RSV vaccine caused a whopping 1,620 cases of “underarm swelling” in axillary lymph nodes:
Even more disappointingly, the vaccine caused 120 long-term axillary swellings, compared to only 49 in the unvaccinated subgroup.
All of this misery and suffering, like 70 vaccine-caused long-term underarm lymph node swellings and 1,620 vaccine-caused total lymph node swellings, was imposed upon the unlucky vaccinated subgroup to prevent a measly 66 cases of RSV.
EDIT: Why Did Placebo Group have so much Lymph Node Swelling?
An astute reader, “Rustam,” asked a good question:
Igor - aside from the vax recipient cohort - how did a massive 1,091 of the "saline placebo" patients end up with underarm swelling?! 1,091 out of 17,679 cohort = 6.17%. Did moderna by chance get unlucky and recruit the world's most unhealthy armpit owners? Or are they lying about the placebo being saline (which sounded a bit too good to be true)?
My answer: Moderna trial participants are mRNA vaccine fanatics and most likely took COVID boosters in addition to the RSV vaccine. That explains why the “placebo” group had so much lymph node swelling, as well as other adverse events.
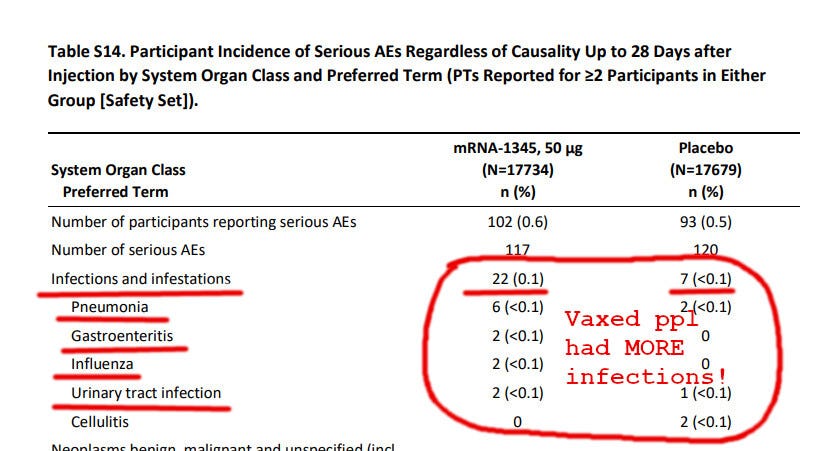
Another mRNA Vaccine Causing Immune Deficiency?
Of particular note is the fact that far more vaccinated subjects had severe “infections and infestations” up to 28 days after vaccination compared to the unvaccinated ones:
Why would mRNA-vaccinated subjects have more pneumonia and other serious illnesses?
Does all that make the proposed mRNA RSV vaccine safe and effective?
Is there a “positive risk-benefit balance”?
Will the corrupt FDA careerists rubber-stamp this ineffective mRNA vaccine, whose only benefit would be enriching Moderna’s shareholders?
Let us know what you think!
Upgrade to paid
''







Yes. The whole mRNA platform needs to be abandoned
Even if the vaccine caused 81 deaths for every case of RSV prevented would that stop a corrupted and captured FDA from approving it?