mRNA data to the FDA and trials by Pfizer and Moderna were frauds? Yes they were "Editor of the British Medical Journal tells the FDA about Serious Concerns over Pfizer Trial Data Integrity"
Serious frauds & we need Pfizer and Moderna investigated and their 2 CEOs Bourla and Bancel properly in proper legal inquiry; short duration of follow-up prevented ANY proper assessment of vax safety
Good piece by nakedemperor.substack.com, support if you can, very good work routinely.
“I have written a few posts about Peter Doshi as he seems to be a man of integrity, reporting on issues that most mainstream publications don’t or won’t. Dr Doshi in an associate professor of pharmaceutical health at the University of Maryland School of Pharmacy, as well as a senior editor at the British Medical Journal. “His research focuses on the drug approval process, how the risks and benefits of medical products are communicated, and improving the credibility and accuracy of evidence synthesis and biomedical publications.”
In the most recent Food and Drug Administration (FDA) Vaccines and Related Biological Products Advisory Committee meeting in the US (6 April 2022), Peter dialled in to the Open Public Hearing Session. This is where members of the public can present their own information to the FDA. The committee was meeting to discuss considerations for the use of COVID-19 vaccine boosters and the process for COVID-19 vaccine strain selection to address current and emerging variants.
Peter told the FDA about Brook Jackson, a whistle-blower from Ventavia, which ran Pfizer’s vaccine trials. He discussed how unblinding of trial participants seems to have occurred and how this creates serious concerns about data integrity. Dr. Doshi also highlighted the lack of FDA inspection. A transcript of his comments is below.
Hi, I’m Peter Doshi, thanks for the opportunity to speak. Hopefully you can see my title slide with my financial disclosures. For identification purposes, I am on the faculty at the University of Maryland and an editor at The BMJ. I have no relevant conflicts of interest and my comments today are my own.
Last November, The BMJ reported the disclosures of a whistle-blower named Brook Jackson, who worked for Ventavia, a contract research company that ran three of the clinical trial sites for Pfizer’s vaccine. Jackson alleged the company had falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events. She provided The BMJ with company emails, internal documents, text messages, photos and recordings of her conversations with company employees.
This photo, for example, shows vaccine packaging materials that are only supposed to be seen by unblinded staff, just left out in the open.
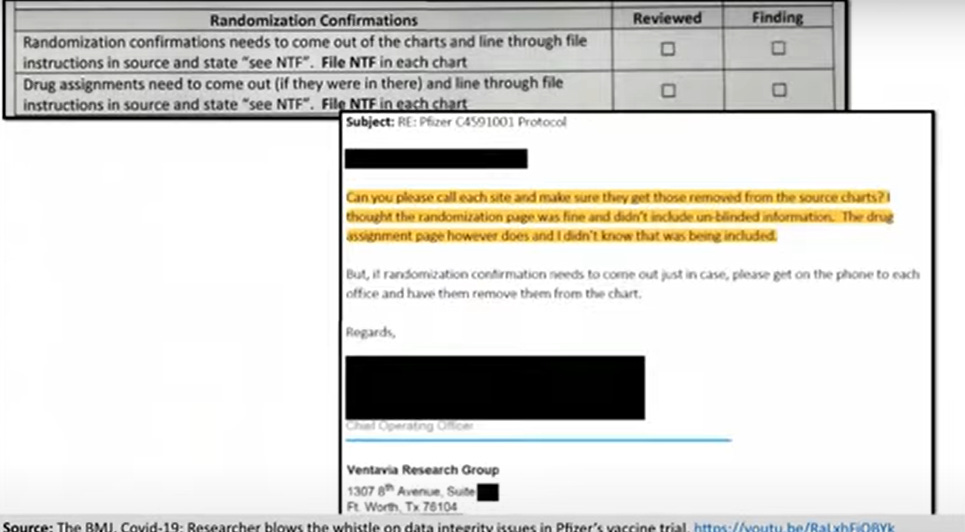
And unblinding may have occurred on a far wider scale. Here you can see the document containing the instructions Ventavia staff were given to file each trial participant’s randomization and drug assignment confirmation sheet into each participant’s chart. This contained unblinded information.
Unblinding, as I think everybody knows, creates serious concerns about data integrity. Once this massive error was discovered, Ventavia asked staff to go through each and every chart to take out the randomization and drug assignment confirmations. You can see here an email from Ventavia’s COO reacting after discovery of the problem: they had not even realized that the drug assignment confirmation contained unblinding information.
In the heat of a pandemic, it’s not hard to imagine that corners were cut and mistakes were made. Some mistakes are benign, but others carry serious consequences to data integrity. One hopes Ventavia is an extreme outlier, but we need more than just hope. We need evidence that the data were dealt with properly. We need regulatory oversight. But despite whistleblower Brook Jackson’s direct complaint to the FDA, FDA never inspected Ventavia. In fact, FDA only inspected 9 of the trial’s 150-plus sites before approving the vaccine. Just 9 sites. And Pfizer continues to use Ventavia for trials.
What about Moderna? FDA had over a year and inspected just one – ONE – of the trial’s 99 sites. How can FDA feel confident in the Moderna data based on a 1% sample?
Data integrity requires adequate regulatory oversight. Trustworthy science requires data transparency. It’s been over a year, but anonymised participant level data remain inaccessible to doctors, researchers, and the public. The public paid for these products, and the public takes on the balance of benefits and harms post vaccination. The public has a right to data transparency, and FDA has an obligation to act. Thank you.
The video for the meeting is below. Peter Doshi’s statement starts at 5:34:44 but all of the public presentations are interesting and these begin at around 5:15.’
SOURCE:






Peter Doshi and the BMJ are one of the few lights of truth in this malignant web of lies.
I can only hope that the criminal activities of EVERYONE involved will eventually have consequences for them! They simply cannot get away with it! There are thankfully more people realizing that the emperor is stark naked!
https://changeandevolve.substack.com/p/the-emperors-new-clothes