'NOVAVAX COVID-19 Vaccine and Myocarditis: New October 2023 papers raise SERIOUS CONCERNS!' Dr. W. Makis raises serious questions about NOVAVAX vaccine & I agree, see both substacks
Reality is it has never been proven (NOVAVAX) safe and now reports are emerging to show it is not safe and it also results in the spike protein that is pathological; all COVID vaccines end in spike
The reality is it has never been proven (NOVAVAX) safe and now reports are emerging to show it is not safe and it also results in the spike protein that is pathological; all COVID vaccines end in spike protein.
see the ‘The “Good News” regarding NOVAVAX and it’s technology:
It’s not an mRNA COVID-19 vaccine
It doesn’t have a Pfizer or Moderna Lipid Nanoparticle
it has a finite quantity of spike protein that is alleged to be 5 ug
No “DNA contamination” (hopefully)
according to the company it has an “acceptable safety profile”
The Bad
It uses Wuhan spike protein
1:1400 died in Novavax Clinical Trials (21 deaths from 30,000 participants)
myocarditis and pericarditis are now considered identified risks for NVX-CoV2373
There were 5 cases of myocarditis/pericarditis in the Novavax Clinical Trials, 4 were “serious” and hospitalized
Company doesn’t know the specific mechanism that causes cardiac tissue inflammation (myocarditis/pericarditis)
Study by Saint-Gerons et al analyzed 61 cases of myocarditis/pericarditis reported to regulators, 62% men
rate of myocarditis similar to Pfizer mRNA COVID-19 vaccines (suggesting nanoparticle involvement)
only 13% recovered
FDA Briefing Document (June 2022)
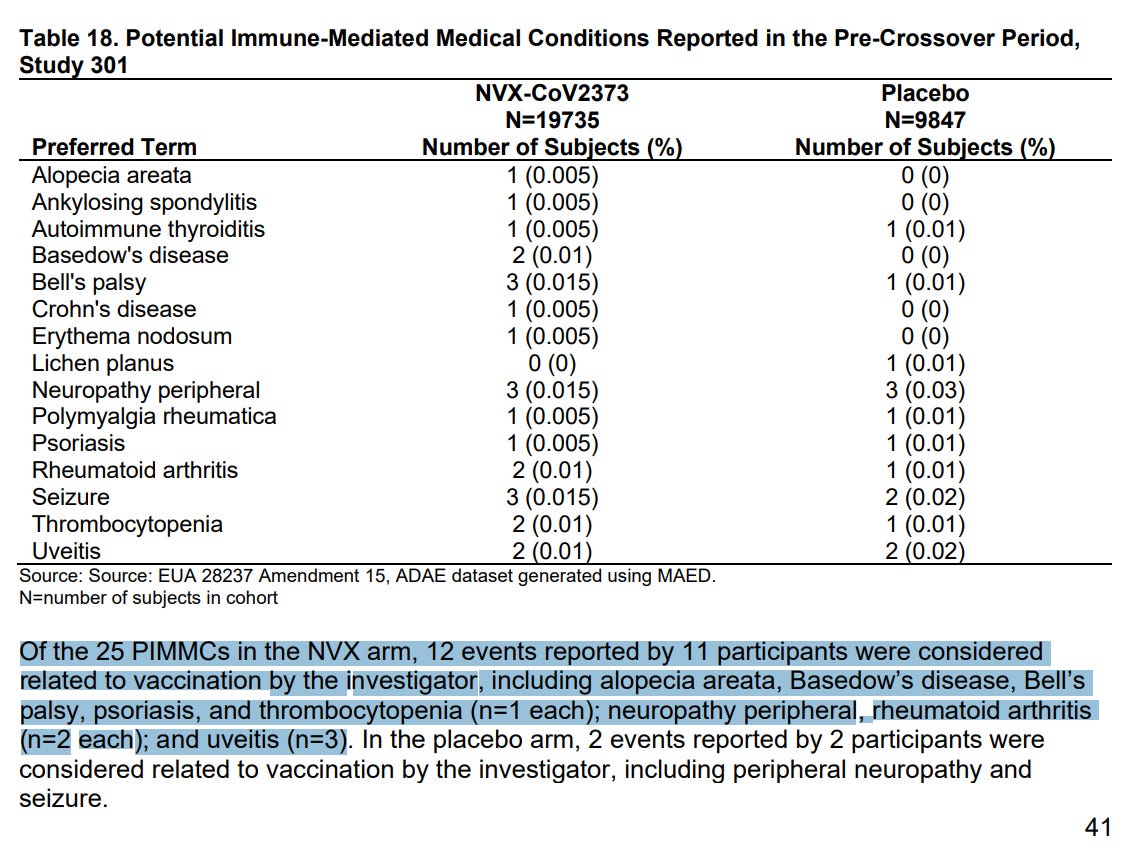
Immune mediated medical conditions related to Novavax: Alopecia, autoimmune thyroid disease, Bell’s Palsy, psoriasis, thrombocytopenia, peripheral neuropathy, rheumatoid arthritis, uveitis (these are all associated with spike protein damage seen with mRNA vaccines after systemic distribution of LNP/mRNA!)
according to FDA, risk of myocarditis after Novavax could be higher than mRNA COVID-19 vaccines
The Ugly
original Novavax is no longer on the market in the US
2023-2024 formulation of Novavax with XBB.1.5 spike protein has no clinical studies evaluating safety or effectiveness
Spike proteins are mixed in a detergent solution where they “self-assemble” around a Polysorbate-80 core
“polysorbate 80 is not an inert compound and has been implicated in a number of systemic and injection- and infusion-site adverse events (ISAEs)”
ALSO contains a saponin-based Matrix-M adjuvant (40nm nanoparticle, remarkably stable, held together by saponin-cholesterol interactions” (in rodents causes decreased body weight and red cell mass) - “may contribute to immunotoxicity”
biodistribution of Novavax nanoparticle-spike has never been done and company “assumes” that it “mostly” stays in local lymph nodes because it should behave like a “conventional protein antigen” (it’s NOT, it’s a nanoparticle)
No studies have been performed to measure nanoparticle-spike persistence in local or distal tissues
“Novavax expects a low potential for serious unwanted effects and toxicities in neonates, infants, and children” (they don’t know)
“Novavax expects a low potential for serious unwanted effects and toxicities in pregnant women and fetuses” (they don’t know)
“Novavax does not believe there are other special populations which would be more susceptible for serious unwanted effects and toxicities” (they don’t know)
CONCLUDING MAKIS THOUGHTS:
Novavax is made of 2 nanoparticles, a 60nm Polysorbate-80/Wuhan-spike rosette and 40nm Matrix-M (saponin-cholesterol)
it seems they got away with not calling Matrix-M a nanoparticle by calling it an “adjuvant” instead, something that’s added to stimulate an immune response, but it’s a nanoparticle.
Matrix-M is a nanoparticle: “The Matrix-M adjuvant consists of two distinct fractions of saponins purified from the Quillaja saponaria Molina tree, combined with cholesterol and phospholipids to form 40-nm open cage-like nanoparticles”
Both are made in detergent solutions, and the Polysorbate-80 core and the Matrix-M are not inert - both could cause immunotoxicity and adverse events - these risks have not been studied
Novavax causes myocarditis comparable to Pfizer, only 13% recover!
Novavax never did a biodistribution study on the Polysorbate-80/Wuhan-spike but it’s proven to get in the bloodstream and go systemic because the FDA Briefing document outlines many immune adverse events all over the body.
Novavax also never did studies on persistence of spike in local or distant tissues and organs.
Novavax - no data on safety in neonates, infants, children
Novavax - no data on safety in pregnancy
11.4% had a Grade 3+ systemic reaction within 7 days with no long term safety data.’
‘Papers Reviewed:
Oct.26, 2023 Wilkinson et al - A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the Novavax COVID-19 Vaccine (NVX-CoV2373), a recombinant spike protein vaccine with Matrix-M adjuvant to prevent disease caused by SARS-CoV-2 viruses
Oct.18, 2023 - COVID-19 Update: New Novavax Vaccine Formulation for 2023-2024
June 2023 Smith et al - Safety of the NVX-CoV2373 COVID-19 vaccine in randomized placebo-controlled clinical trials
Feb.2023 Saint-Gerons et al - Myopericarditis Associated with the Novavax COVID-19 Vaccine (NVX-CoV2373): A Retrospective Analysis of Individual Case Safety Reports from VigiBase
Dec.2022 Ahmad et al - Myopericarditis following both BNT162b2 and NVX-CoV2373
June 7 2022 - U.S. Food and Drug Administration. Novavax COVID-19 vaccine (NVX-CoV2373) VRBPAC briefing document. Updated 2022
Oct.26, 2023 Wilkinson et al - A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the Novavax COVID-19 Vaccine (NVX-CoV2373), a recombinant spike protein vaccine with Matrix-M adjuvant to prevent disease caused by SARS-CoV-2 viruses
“Novavax, Inc. has developed a recombinant protein vaccine formulated with the saponin-based Matrix-M™ adjuvant (referred to as NVX-CoV2373) against SARS-CoV-2”
“SARS-CoV-2 recombinant spike (rS) nanoparticle consists of a highly purified protein constructed from the full-length, wild-type SARS-CoV-2 spike. This protein is based on the spike gene sequence from the original Wuhan-Hu-1 strain and is produced using a baculovirus expression system in an insect cell line that is derived from Sf9 cells of the Spodoptera frugiperda species”
“Notably, the flexibility of this step allows for the use of alternative genetic constructs as needed, such as the S-proteins from new SARS-CoV-2 variants”
“recombinant spike protein was modified to contain amino acid substitutions in the S1/S2 furin cleavage and two proline substitutions in the S2 domain to confer protease resistance and to produce a stable prefusion conformation”
“the recombinant baculovirus is replicated in Sf9 cells and NVX-CoV2373 spike gene is transcribed, translated, and post-translationally modified (i.e. glycosylated), resulting in high-yield production of the recombinant SARS-CoV-2 S-protein in a native trimer conformation. Next, the trimers are harvested, purified by column chromatography and filtration, and dialyzed into a detergent-containing buffer solution wherein they self-assemble into ~ 60 nanometer particles arranged around a polysorbate 80 core.”
In the final step, the S-protein nanoparticles are formulated with Matrix-M saponin-based adjuvant (Matrix-MTM)
“formulation with Matrix-M, a potent and stable adjuvant studied in over 37,000 individuals, allows for antigen dose sparing and enhanced immune responses while maintaining a favorable safety profile”
In over 40 countries, NVX-CoV2373 is either authorized for emergency use or has received full approval
SAFETY: “NVX-CoV2373 safety has been assessed throughout the comprehensive non-clinical and clinical development programs and in post authorization safety surveillance. No risks were identified in the nonclinical development program while data supported the dose and regimen approved for human use. The NVX-CoV2373 safety profile indicates this vaccine is well tolerated as demonstrated by data from over 31,000 participants receiving NVX-CoV2373 across 5 randomized controlled clinical trials.”
Novavax = 5 ug (Wuhan strain spike protein) + 50 ug Matrix-M
“there is paucity of evidence about the longevity of the antibody response to COVID-19 infection…therefore, reinfection with COVID-19 is possible”
The Matrix-M adjuvant is manufactured by mixing defined, partially purified extracts of the bark of the Quillaja saponaria Molina tree, termed Fraction A and Fraction C, with cholesterol and phosphatidylcholine in the presence of a detergent.
The resulting spherical immune stimulating complexes are particles of approximately 40 nm diameter, are remarkably stable, held together by the strong affinity between saponin and cholesterol. The particle also provides chemical stability to the fragile saponin molecules under conditions where free saponins degrade quickly.Matrix-M adjuvant administration was generally well tolerated in humans and animals. Toxicology data from animal studies to evaluate Matrix-M adjuvant, alone or co-administered with different vaccine antigens, did not demonstrate relevant systemic or organ-specific toxicities. There were transient and inconsistent reductions in body weight and red cell mass parameters
Matrix-M has been tested with Influenza, RSV and Ebola nanoparticle vaccines
“immunostimulation from Matrix-M adjuvant may also contribute to immunotoxicity”
biodistribution studies of Matrix-M have been done in rodents
biodistribution of nanoparticle-spike hasn’t been done - WHY? - “We anticipate that the co-formulated vaccine will follow a conventional protein antigen trafficking pathway following intramuscular administration: the antigen taken up by dendritic cells will migrate to the draining lymph node where it is processed and presented to T cells by antigen-presenting cells and initiate the immune response. Therefore, we expect that detectable antigen distribution would mostly be limited to local lymph nodes”
No studies have been performed to measure persistence in local or distal tissues due to the very low microgram doses of antigen, it will be difficult to detect in serum and tissues.
delivery in the context of a saponin adjuvant is designed to facilitate transport to the draining lymph nodes rather than formation of a local depot.
“Novavax expects a low potential for serious unwanted effects and toxicities in neonates, infants, and children”
“Novavax expects a low potential for serious unwanted effects and toxicities in pregnant women and fetuses”
“Novavax does not believe there are other special populations which would be more susceptible for serious unwanted effects and toxicities”
Oct.18, 2023 - COVID-19 Update: New Novavax Vaccine Formulation for 2023-2024
original formulation of Novavax vaccine is no longer authorized for use in US
2023-2024 formulation of the Novavax vaccine contains the spike protein of the XBB.1.5 Omicron strain of SARS-CoV-2.
No clinical studies evaluating the SAFETY or effectiveness of the 2023-2024 Novavax vaccine are available
“The updated Novavax COVID-19 vaccine is indicated for use in persons ≥12 years old who have not already received another 2023-2024 COVID-19 vaccine formulation”
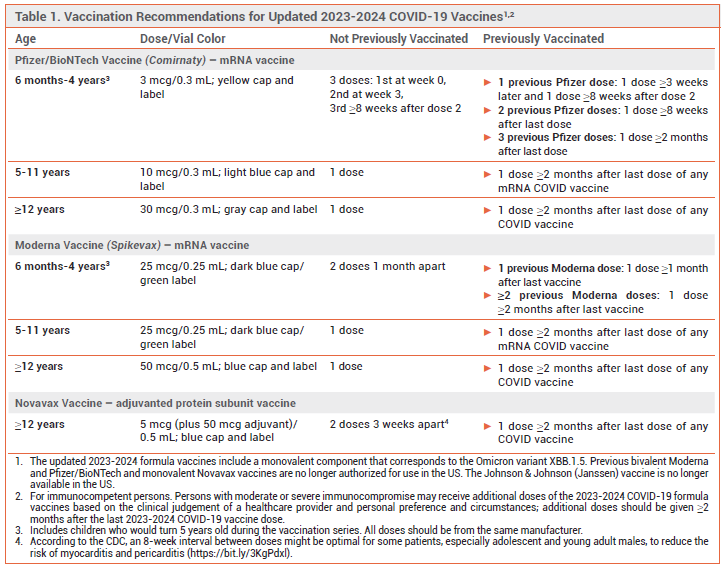
WARNING: “Those who have not previously been vaccinated against COVID-19 should receive 2 doses; the product labeling recommends that they be given 3 weeks apart, but according to the CDC, an 8-week interval between doses might be optimal for some patients (especially adolescent and young adult males) to reduce the risk of myocarditis and pericarditis”
June 2023 Smith et al - Safety of the NVX-CoV2373 COVID-19 vaccine in randomized placebo-controlled clinical trial
4 Clinical Trials with 30,058 getting Novavax, 19,892 getting placebo
11.4% had a Grade 3+ systemic reaction within 7 days (3.6% for placebo)
21 deaths or 0.07% (12 deaths or 0.06% in placebo)
“Grade 3–4 events were more frequent in vaccine recipients than placebo recipients, which is not unexpected for an adjuvanted vaccine compared to a saline placebo. SAE rates reported by participants who received NVX-CoV2373 or placebo were similar”
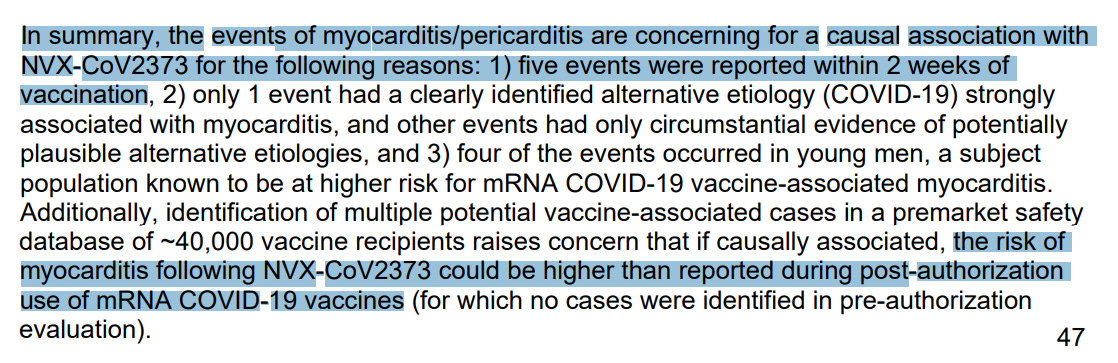
“Following marketing authorization of NVX-CoV2373, reports of myocarditis and pericarditis were received from several regions, including Australia and the European Union. Based on a review of the cumulative post-authorization reports, myocarditis and pericarditis are now considered identified risks for NVX-CoV2373”
“It is unclear what specific mechanism causes the cardiac tissue inflammation.”
Feb.2023 Saint-Gerons et al - Myopericarditis Associated with the Novavax COVID-19 Vaccine (NVX-CoV2373): A Retrospective Analysis of Individual Case Safety Reports from VigiBase
61 safety reports for myocarditis and pericarditis reported to regulators, 24 serious, 19 required hospitalization, 3 were life threatening, 1 disabling, none were fatal
median age 36 years old, 62%, 38% women
70% had chest pain
median period to onset 3 days after jab
only 13% recovered
“disproportionality signal was found for NVX-CoV2373 (Novavax) vaccine in line with the mRNA vaccines, and the Pfizer-BioNTech vaccine in particular”
AstraZeneca had elevated risk of myocarditis but only 1/10th that of Pfizer or Novavax.
“More research would be needed to understand the role of nanoparticles in the potential risk of vaccine-induced myocarditis.”
Interpretation: Novavax has myocarditis risk comparable to Pfizer mRNA
Dec.2022 Ahmad et al - Myopericarditis following both BNT162b2 and NVX-CoV2373
This case report presents 2 cases of pericarditis and myocarditis recurrence within 1 week following booster dose vaccination with NVX-CoV2373
both cases has similar clinical presentations:
both subjects had mirroring events following their BNT162b2 and NVX-CoV2373 vaccinations
As young individuals who had reactions within 7 days of a non-first dose vaccination, both also fit the same demographic usually seen in post-mRNA vaccine myopericarditis
Both fulfill the criteria of myopericarditis, with presentations of pleuritic chest pain and ECG
Theory:
Pfizer myocarditis - immunogenicity of the lipid nano particle (LNP) sheath required to deliver mRNA to host cells - potential cause for either direct damage to the myocardial cells, or as another trigger for immune dysregulation.
nanoparticles are also used in the NVX-CoV2373 vaccine, which is required for incorporation of the S-protein into the host
June 7 2022 - U.S. Food and Drug Administration. Novavax COVID-19 vaccine (NVX-CoV2373) VRBPAC briefing document. Updated 2022
My Take…
The “Good News”
It’s not an mRNA COVID-19 vaccine
It doesn’t have a Pfizer or Moderna Lipid Nanoparticle
it has a finite quantity of spike protein that is alleged to be 5 ug
No “DNA contamination” (hopefully)
according to the company it has an “acceptable safety profile”
The Bad
It uses Wuhan spike protein
1:1400 died in Novavax Clinical Trials (21 deaths from 30,000 participants)
myocarditis and pericarditis are now considered identified risks for NVX-CoV2373
There were 5 cases of myocarditis/pericarditis in the Novavax Clinical Trials, 4 were “serious” and hospitalized
Company doesn’t know the specific mechanism that causes cardiac tissue inflammation (myocarditis/pericarditis)
Study by Saint-Gerons et al analyzed 61 cases of myocarditis/pericarditis reported to regulators, 62% men
rate of myocarditis similar to Pfizer mRNA COVID-19 vaccines (suggesting nanoparticle involvement)
only 13% recovered
FDA Briefing Document (June 2022)
Immune mediated medical conditions related to Novavax: Alopecia, autoimmune thyroid disease, Bell’s Palsy, psoriasis, thrombocytopenia, peripheral neuropathy, rheumatoid arthritis, uveitis (these are all associated with spike protein damage seen with mRNA vaccines after systemic distribution of LNP/mRNA!)
according to FDA, risk of myocarditis after Novavax could be higher than mRNA COVID-19 vaccines
The Ugly
original Novavax is no longer on the market in the US
2023-2024 formulation of Novavax with XBB.1.5 spike protein has no clinical studies evaluating safety or effectiveness
Spike proteins are mixed in a detergent solution where they “self-assemble” around a Polysorbate-80 core
“polysorbate 80 is not an inert compound and has been implicated in a number of systemic and injection- and infusion-site adverse events (ISAEs)”
ALSO contains a saponin-based Matrix-M adjuvant (40nm nanoparticle, remarkably stable, held together by saponin-cholesterol interactions” (in rodents causes decreased body weight and red cell mass) - “may contribute to immunotoxicity”
biodistribution of Novavax nanoparticle-spike has never been done and company “assumes” that it “mostly” stays in local lymph nodes because it should behave like a “conventional protein antigen” (it’s NOT, it’s a nanoparticle)
No studies have been performed to measure nanoparticle-spike persistence in local or distal tissues
“Novavax expects a low potential for serious unwanted effects and toxicities in neonates, infants, and children” (they don’t know)
“Novavax expects a low potential for serious unwanted effects and toxicities in pregnant women and fetuses” (they don’t know)
“Novavax does not believe there are other special populations which would be more susceptible for serious unwanted effects and toxicities” (they don’t know)
CONCLUDING THOUGHTS:
Novavax is made of 2 nanoparticles, a 60nm Polysorbate-80/Wuhan-spike rosette and 40nm Matrix-M (saponin-cholesterol)
it seems they got away with not calling Matrix-M a nanoparticle by calling it an “adjuvant” instead, something that’s added to stimulate an immune response, but it’s a nanoparticle.
Matrix-M is a nanoparticle: “The Matrix-M adjuvant consists of two distinct fractions of saponins purified from the Quillaja saponaria Molina tree, combined with cholesterol and phospholipids to form 40-nm open cage-like nanoparticles”
Both are made in detergent solutions, and the Polysorbate-80 core and the Matrix-M are not inert - both could cause immunotoxicity and adverse events - these risks have not been studied
Novavax causes myocarditis comparable to Pfizer, only 13% recover!
Novavax never did a biodistribution study on the Polysorbate-80/Wuhan-spike but it’s proven to get in the bloodstream and go systemic because the FDA Briefing document outlines many immune adverse events all over the body.
Novavax also never did studies on persistence of spike in local or distant tissues and organs.
Novavax - no data on safety in neonates, infants, children
Novavax - no data on safety in pregnancy
11.4% had a Grade 3+ systemic reaction within 7 days with no long term safety data.





THERE IS NO COVID VIRUS, THE WHOLE THING WAS TO GET YOU INJECTED WITH THE BIO-WEAPON Clot-Shots to genocide the population. They have never isolated the Covid Virus.
They put Graphene Hydroxide in the "vaccine" which has nano-scale "razor-blades" that tear up the epithelial lining of the blood vessels and organs causing things to stick to the tears of the lining and form blood clots which causes the myocarditis and pericarditis and finally death.
The foremost expert on this finding was murdered for exposing these findings. His name is Dr. Andreas Noack and a video which he made 4 days before his death follows.
GRAPHENE "RAZOR BLADES" FOUND IN THE COVID VACCINES (DR. ANDREAS NOACK)
https://www.bitchute.com/video/X9oMvf6dbhCi/
Why in the world would we need any of the vaxes they offer? Just say NO!