REMDESIVIR! JM Phelps writes in the Gateway Pundit: 'The Remdesivir Papers: Did Service Members Deserve to Die?' see his excellent article & my inserts showing how Fauci & NIH committed fraud by
changing the primary patient-important endpoints of death etc. and made a secondary UNIMPORTANT outcome the key driver, changing the goalpost to make it seem like the drug was beneficial, effective
My inserts for I show here the actual protocol fraud by NIH and Fauci, note also they disregarded the Wang et al. LANCET study out the very same day that showed that Remdesivir FAILED! Was harmful!
ssshhhh, but can someone ask Robert Malone what he knows about this? Better yet, not yet, let us wait until he is under oath…
'The Remdesivir Papers: Did Service Members Deserve to Die?'
Writing for American Thinker, author Stella Paul once said, “Remdesivir may be the most despised drug in American history, earning the nickname ‘Run Death Is Near’ for its lethal record during COVID.”
The Remdesivir Papers captures the essence of her statement for service members and veterans across the country.
Data derived from the Department of Defense Joint Trauma System (JTS) by a military whistleblower offers a stark contrast to the results of multiple clinical trials involving the liberal usage of remdesivir in military treatment facilities and other civilian facilities, as well as its potential contribution to, at minimum, hundreds of untimely deaths.
Interviews with that whistleblower indicate the trials surrounding the use of remdesivir and respective protocols for the treatment of COVID-19 either (1) provided half-truths or (2) kept their secrets close. Big Pharma played their part, and the Department of Defense bought it. Either the U.S. government blindly fell for all of it, considering remdesivir an acceptable treatment for COVID-19 despite a lack of evidence … or it opened itself to investigation.
“Gilead is working closely with global health authorities to respond to the novel coronavirus (2019-nCoV) outbreak through the appropriate experimental use of our investigational compound remdesivir. Together with the U.S. Food and Drug Administration (FDA), the U.S. Centers for Disease Control and Prevention (CDC), the U.S. Department of Health and Human Services (DHHS), the U.S. Department of Defense (DOD) – CBRN Medical, the China CDC and National Medical Product Administration (NMPA), the World Health Organization (WHO), and the U.S. National Institute of Allergies and Infectious Diseases (NIAID), and along with individual researchers and clinicians, Gilead is focused on contributing our antiviral expertise and resources to help patients and communities fighting 2019-nCoV.”
With a patent valid for up to 20 years, Gilead had already created a monopoly on the drug in 2017. Interestingly, in the same year, two researchers from the University of North Carolina Gillings School of Global Public Health were awarded more than $6 million to “accelerate the development of a promising new drug (remdesivir) in the fight against deadly coronaviruses.”
The researchers discovered that the development of severe acute respiratory syndrome coronavirus (SARS-CoV) in mice could be prevented by remdesivir (GS-5734TM).
Although the investigational drug was developed by Gilead Sciences as a treatment for the Ebola virus in 2017, it was subsequently proven to be lethal in human patients with the virus in 2018. Curiously, in February 2020, remdesivir – despite its deadly history – was approved for use in clinical trials to evaluate its efficacy in the treatment of COVID-19.
Published by The New England Journal of Medicine in November 2019, the use of remdesivir resulted in the highest mortality rate among participants in a trial of four investigational therapies for Ebola in the Democratic Republic of Congo.
Despite the deaths of 93 out of 175 (53.1 percent) of patients, the broad-spectrum antiviral was eagerly considered for a possible COVID-19 treatment.

Shortly thereafter, at a Defense Department press briefing on March 5, 2020, Army Brig. Gen. Michael J. Talley, commanding general of U.S. Army Medical Research and Development Command (USAMRDC) and Fort Detrick, revealed that “a cooperative research and development agreement with an industry partner is under review for the DOD to gain access to an antiviral drug for treatment use in our medical centers, our military treatment facilities.”
Five days later, it was announced that Fort Detrick’s U.S. Army Medical Materiel Development Activity (USAMMDA), a subordinate command of USAMRDC, had entered into a Cooperative Research and Development Agreement (CRADA) with the American biopharmaceutical company Gilead Sciences.
Headquartered in Foster City, California, Gilead agreed to provide remdesivir for the treatment of DOD personnel exposed to severe acute respiratory syndrome coronavirus (SARS-CoV-2), the causative viral pathogen of COVID-19.
“The trust and hope placed in our team by the Department of Defense, the Department of the Army and the American public have already enabled us to make great strides in this fight, and we are eager to pursue this effort to the end,” said Fort Detrick’s Brig. Gen. Talley.
A March 11, 2020 press release by USAMMDA shared that “in the CRADA, the USAMMDA Force Health Protection program will allow for the investigational use of remdesivir provided by Gilead, at no cost to the government, in the absence of any approved treatment options.”
In an April 2020 news report, Quartz stated very effectively: “News of the military’s deal with Gilead was surprising, shining a light on the military’s unique ability to acquire medications before the FDA has signed off on the same drug for average Americans, if it ever does.”
According to a statement provided to Quartz by the USAMMDA, “Remdesivir was chosen because it was the most mature, broad spectrum antiviral drug in development and showed activity in vitro and in animal models in decreasing the viral replication against coronaviruses.”
Full Speed Ahead
Alongside heightened interest by the military and its purported promise, remdesivir moved quickly through government scrutiny. The FDA issued an Emergency Use Authorization (EUA) for remdesivir on May 1, 2020 – less than two months after the CRADA with Gilead was announced.
In August, a press release by Gilead Sciences announced the submission of a New Drug Application (NDA) to the FDA for remdesivir under the name Veklury®. An NDA is “the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the U.S.”
Near the end of May, The Lancet – one of the world’s leading peer-reviewed journals – published the results of “a randomised, double-blind, placebo-controlled, multicentre trial” that occurred in China between February 6, 2020, and March 12, 2020.
In summary, that trial brought concerns about the efficacy of remdesivir. While it has been suggested that the trial was stopped because “the epidemic of COVID-19 has been controlled well in China [and] no eligible patients can be enrolled at present,” the authors of the study said, “remdesivir was stopped early because of adverse events in 18 (12%) patients versus four (5%) patients who stopped placebo early.”
This begs a question with regard to the use of remdesivir: Did those conducting the study conclude that adverse events would have continued to more than double if the trial had continued?
With an inability to demonstrate efficacy accompanied by stock sliding in Gilead between 2015 and 2020, it’s interesting to note that “the U.S. has bought up virtually all the stocks [of remdesivir] … leaving none for the U.K., Europe or most of the rest of the world,” according to an April 2020 report by The Guardian.
Six months later, in October 2020, remdesivir (Veklury®) was approved by the FDA as the “first treatment for COVID-19” for certain populations of adult and pediatric patients.
For remdesivir to be approved, the Federal Food, Drug, and Cosmetic Act required “substantial evidence of effectiveness and a demonstration of safety for the drug’s intended use(s).”
In considering a drug’s approval, the FDA “conducts a benefit-risk assessment based on rigorous scientific standards to ensure that the product’s benefits outweigh its risks for the intended population.”
For this reason, FDA Commissioner Stephen M. Hahn, M.D. affirmed, “[the] approval [of remdesivir] is supported by data from multiple clinical trials that the agency has rigorously assessed and represents an important scientific milestone in the COVID-19 pandemic.”
For some observers, the FDA’s rapid approval of remdesivir didn’t pass muster. For example, Vox’s Umair Irfan wrote, “the FDA is once again promoting a Covid-19 therapy based on shaky evidence, [expressing that] researchers are concerned the FDA’s first full approval of a Covid-19 drug doesn’t have enough research behind it.”
In 2021, Robert F. Kennedy Jr., explained in his book The Real Anthony Fauci (p. 65) that Dr. [Anthony] Fauci “[moved] the goal posts” and created new endpoints to “[allow] the drug to demonstrate a benefit.”
Failing to provide the data necessary to prove the efficacy of the drug, the former chief medical advisor to then-president Donald Trump had asserted remdesivir showed “quite good news” and set a new standard of care for COVID-19 patients.
And initially the World Health Organization (WHO) took issue with the FDA’s decision, issuing “a conditional recommendation [in November 2020] against the use of remdesivir in hospitalized patients, regardless of disease severity, as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.”
In the same month, a WHO trial determined that “the big story is the finding that remdesivir produces no meaningful impact on survival.”
But roughly a year and a half later, the United Nations intergovernmental agency reversed course to “[suggest] the use of remdesivir in mild or moderate COVID-19 patients who are at high risk of hospitalization.”
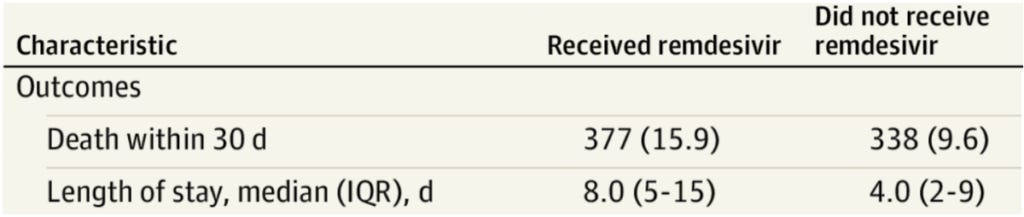
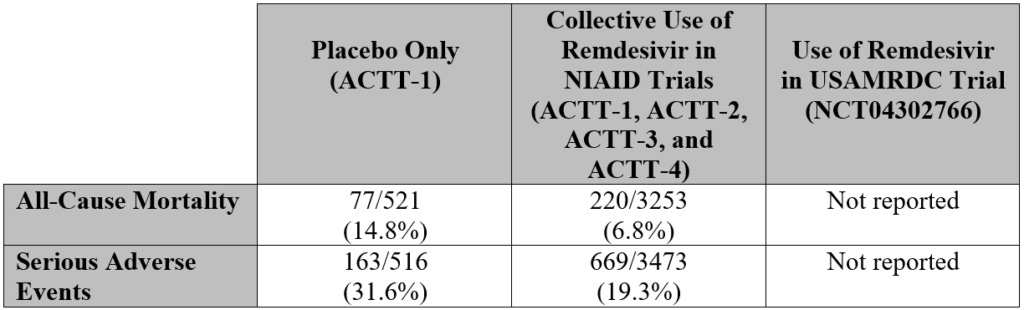
In a cohort study of over 2,300 veterans hospitalized with COVID-19, JAMA Network Open – a medical journal published by the American Medical Association – determined “remdesivir treatment was not associated with survival,” determining that there more deaths among those veterans who received remdesivir than those who did not (see table below). The length of hospital stay was also doubled among those who received remdesivir.
Further eroding the efficacy of remdesivir, in December 2021 – and again on September 23, 2024 – Gilead confirmed the presence of glass particles in lots of remdesivir … demonstrating that not only was the drug poorly formulated, it was poorly manufactured as well.
Clinical Trial Observations
Corroborated at ClinicalTrials.gov, a searchable registry and results database hosted by the National Library of Medicine, service members and veterans participated in at least four Phase 3 Adaptive COVID19 Treatment Trials (ACTT) between February 2020 and June 2021, to include NCT04280705 [ACTT-1], NCT04401579 [ACTT-2], NCT04492475 [ACTT-3], and NCT04640168 [ACTT-4]. Each of these trials was sponsored by the National Institute of Allergy and Infectious Diseases (NIAID).
According to the FDA, Phase 3 trials “demonstrate whether or not a product offers a treatment benefit to a specific population…[and] the results are more likely to show long-term or rare side effects.”
Another trial (NCT04302766) was sponsored by U.S. Army Medical Research and Development Command (USAMRDC). While the actual study start and study completion dates are not available at ClinicalTrials.gov, information was first submitted to the registry and database in March 2020.
NCT04280705 [ACTT-1] was conducted at 60 locations, involving 1,062 participants. Participating military locations included Naval Medical Center San Diego, Naval Hospital Jacksonville, Benning Martin Army Community Hospital, Eisenhower Army Medical Center, Tripler Army Medical Center, Blanchfield Army Community Hospital, Walter Reed National Military Medical Center, Naval Medical Center Camp Lejeune, Womack Army Medical Center, William Beaumont Army Medical Center, Carl R. Darnall Army Medical Center, Brooke Army Medical Center, Naval Medical Center Portsmouth, and Madigan Army Medical Center.
Each participant met the criteria of being “admitted to a hospital with symptoms suggestive of COVID-19 infection … [with] laboratory-confirmed SARS-CoV-2 infection as determined by polymerase chain reaction (PCR) or other commercial or public health assay in any specimen.”
The placebo comparator group received “200 mg of remdesivir placebo administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir placebo while hospitalized for up to a 10 days total course.”
The experimental group received “200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir while hospitalized for up to a 10 days total course.”
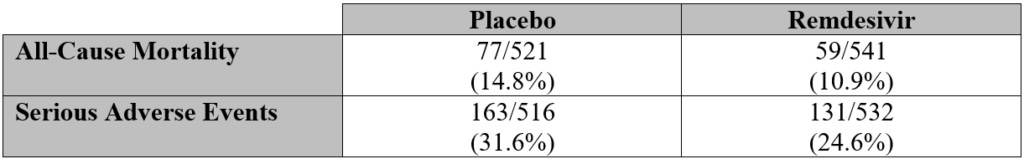
This study had wide inclusion criteria and minimal exclusion criteria. A true placebo, like saline, wasn’t used other than in circumstances where there were limitations on matching placebo supplies.
The study makes no distinction as to who received which placebo. Furthermore, the original enrollment count was used in all result calculations which ignored those who didn’t finish the trial for various reasons, including adverse events.
ADVERTISEMENT
Adjusted for those who didn’t finish the trials (13 placebo and 24 Remdesivir), All-Cause Mortality for the placebo group would have fallen between 12.2 and 17.2 percent. For the remdesivir group, the percentage would have fallen between 6.4 and 15.3 percent.
Overall, this study is problematic due to multiple confounding variables that appear to exist intentionally to present a positive outcome for the remdesivir group.
As an example, if every “All-Cause Mortality” in the placebo group was due, in part, to mechanical ventilation it could still be made to appear that the placebo was to blame.
This study ended up being the only comparison between remdesivir and a type of placebo. A true safety profile couldn’t be established by design and only enabled follow-on studies pairing remdesivir with other investigational drugs.
NCT04401579 [ACTT-2] was conducted at 71 locations, involving 1,033 participants. Participating military locations included VA Palo Alto Health Care System, Naval Medical Center San Diego, Atlanta VA Medical Center, Southeast Louisiana Veterans Health Care System, Walter Reed National Military Medical Center, Womack Army Medical Center, Brooke Army Medical Center, Naval Medical Center Portsmouth, and Madigan Army Medical Center.
ADVERTISEMENT
Each participant met the criteria of being “admitted to a hospital with symptoms suggestive of COVID-19 infection … [with] laboratory-confirmed SARS-CoV-2 infection as determined by polymerase chain reaction (PCR) or other commercial or public health assay in any specimen.”
The placebo comparator group received “200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir while hospitalized for up to a 10-day total course and 4 mg (2 tablets of 2 mg) of Baricitinib Placebo administered orally daily for the duration of the hospitalization up to a 14-day total course.”
The experimental group received “200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir while hospitalized for up to a 10-day total course and 4 mg (2 tablets of 2 mg) of Baricitinib administered orally daily for the duration of the hospitalization up to a 14-day total course.”
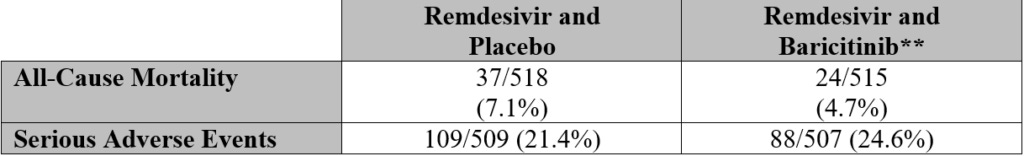
This is representative of a “Remdesivir and” study with no true placebo group which confounds results. Interestingly, 59 participants didn’t finish the trial due to death, which isn’t characterized as being caused by the investigative treatments.
Additionally, no data is presented specific to pre-existing conditions or comorbidities apart from those present in the exclusion criteria (if followed). It’s worth noting that the exclusion criteria expanded to include individuals with liver and kidney conditions.
NCT04492475 [ACTT-3] was conducted at 64 locations, involving 969 participants. Participating military locations included VA Palo Alto Health Care System, Naval Medical Center San Diego, Atlanta VA Medical Center, Tripler Army Medical Center, Southeast Louisiana Veterans Health Care System, Walter Reed National Military Medical Center, Womack Army Medical Center, Brooke Army Medical Center, Naval Medical Center Portsmouth, and Madigan Army Medical Center.
Each participant met the criteria of being “admitted to a hospital with symptoms suggestive of COVID-19 infection … [with] laboratory-confirmed SARS-CoV-2 infection as determined by polymerase chain reaction (PCR) or other commercial or public health assay in any respiratory specimen or saliva.”
The placebo comparator group received “200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir while hospitalized for up to a 10-day total course and a 0.5 mL placebo injection administered subcutaneously on Days 1, 3, 5, and 7 while hospitalized for a total of 4 doses.”
The experimental group received “200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of Remdesivir while hospitalized for up to a 10-day total course and 44 mcg of interferon beta-1a administered by a 0.5 mL subcutaneous injection on Days 1, 3, 5, and 7 while hospitalized for a total of 4 doses.”

Again, this is representative of a “Remdesivir and” study with no true placebo group which confounds results. Additionally, no data is presented specific to pre-existing conditions or comorbidities apart from those present in the exclusion criteria (if followed).
NCT04640168 [ACTT-4] was conducted at 72 locations, involving 1,010 participants. Participating military locations included Palo Alto Health Care System, Naval Medical Center San Diego, VA Eastern Colorado Health Care System, Atlanta VA Medical Center, Tripler Army Medical Center, Walter Reed National Military Medical Center, Womack Army Medical Center, Brooke Army Medical Center, Naval Medical Center Portsmouth, and Madigan Army Medical Center.
Each participant met the criteria of being “hospitalized with symptoms suggestive of COVID-19 infection … [with] laboratory-confirmed SARS-CoV-2 infection as determined by polymerase chain reaction (PCR) or other commercial or public health assay.”
One experimental group received “200 mg of remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of remdesivir while hospitalized for up to a 10-day total course; 4 mg of baricitinib administered as 2 tablets taken orally daily while hospitalized for up to a 14-day total course; and dexamethasone placebo administered as an intravenous injection daily while hospitalized for up to a 10-day total course.”
Another experimental group received “200 mg of remdesivir administered intravenously on Day 1, followed by a 100 mg once-daily maintenance dose of remdesivir while hospitalized for up to a 10-day total course; baricitinib placebo administered as 2 tablets taken orally daily while hospitalized for up to a 14-day total course; and 6 mg of dexamethasone administered as an intravenous injection daily while hospitalized for up to a 10-day total course.”

This is also representative of a “Remdesivir and” study with no true placebo group which confounds results. Additionally, no data is presented specific to pre-existing conditions or comorbidities apart from those present in the exclusion criteria (if followed).
Sponsored by USMARDC, NCT04302766 was conducted at 22 locations (all military facilities), involving an undisclosed number of participants. Participating military locations included Naval Medical Center San Diego, Naval Hospital Jacksonville, Benning Martin Army Community Hospital, Eisenhower Army Medical Center, Tripler Army Medical Center, Blanchfield Army Community Hospital, Walter Reed National Military Medical Center, Naval Medical Center Camp Lejeune, Womack Army Medical Center, William Beaumont Army Medical Center, Carl R. Darnall Army Medical Center, Brooke Army Medical Center, Naval Medical Center Portsmouth, Madigan Army Medical Center, and others abroad.
Each participant met the criteria of being “DoD-affiliated personnel as defined in DoDI 6200.02, which includes emergency-essential civilian employees and/or contractor personnel accompanying the Armed Forces who are subject to the same health risk as military personnel” … and “[having] a laboratory-confirmed COVID-19 diagnosis with moderate to severe disease presentation as determined by the principal investigator.” This study used the broadest inclusion criteria and minimal exclusion criteria in comparison to other investigational studies.
While the trial examined the effects of remdesivir on the treatment of COVID-19, there are no details available at ClinicalTrials.gov to determine the amount of remdesivir administered to participants. There are no results posted to determine all-cause mortality or serious adverse events.
Run Death Is Near
The table below compares the placebo-only trial, the collective use of remdesivir in NIAID trials, and remdesivir use in the USAMRDC trial. For the NIAID trial column, figures were derived by adding each instance of all-cause mortality, as well as serious adverse events, from each of the four trials summarized.
In four of five trials, the use of remdesivir resulted in a minimal positive effect on instances of all-cause mortality and serious adverse side events when compared to the administration of a placebo.
But what about the USAMRDC trial? Did the use of remdesivir in this trial result in a similar positive effect as it was reported in the other trials? Several questions need to be answered:
How many participants were there in the trial?
How many participants died in the trial?
For those who died, what did the participants die from?
How much remdesivir did participants receive? Was it 200 mg of remdesivir administered intravenously on day 1, followed by a 100 mg once-daily maintenance for 10 days? Or was there another treatment protocol offered?
What were the results of the trial?
Were some of the deaths attributed to COVID-19 then they could/should have been attributed to remdesivir or treatment protocol?
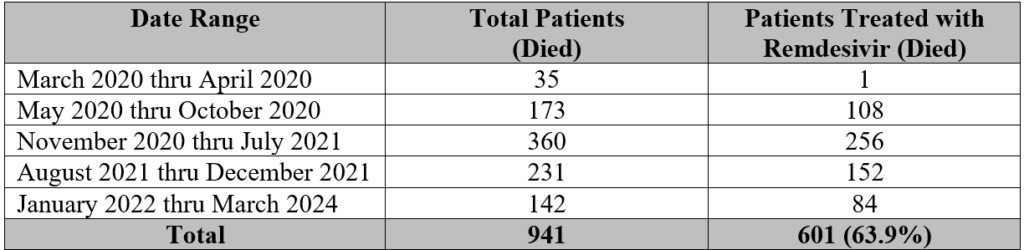
It’s important that these questions be answered because data derived from the DOD’s Joint Trauma System (JTS) by a military whistleblower offers a stark contrast to results of the other trials. That JTS data (shown in the following table) clearly depicts the liberal usage of remdesivir in military treatment facilities (MTFs) and other civilian facilities, as well as its potential contribution to, at minimum, hundreds of untimely deaths.
Shockingly, 64 percent of patients who died between March 2020 and March 2024 were treated with remdesivir. Interestingly, the World Health Organization reported in 2020 that only “about 3.4% of reported COVID-19 cases have died.” Two years later, research has determined the infection fatality rate (IFR) had a median of “0.002% at 20-29 years, 0.011% at 30–39 years, 0.035% at 40-49 years, 0.123% at 50-59 years, and 0.506% at 60-69 years.”
Another study, published by BMC Infectious Diseases, determined that “the risk of severe and critical disease increases exponentially with age, but much less steeply than the risk of fatal illness.”
While the military is only a subset of the larger population, it’s clear that service members and veterans who contracted severe COVID and were subsequently treated with remdesivir had a much higher chance of dying. Until results are shared publicly, there is no evidence of a positive effect to found in the USAMRDC trial.
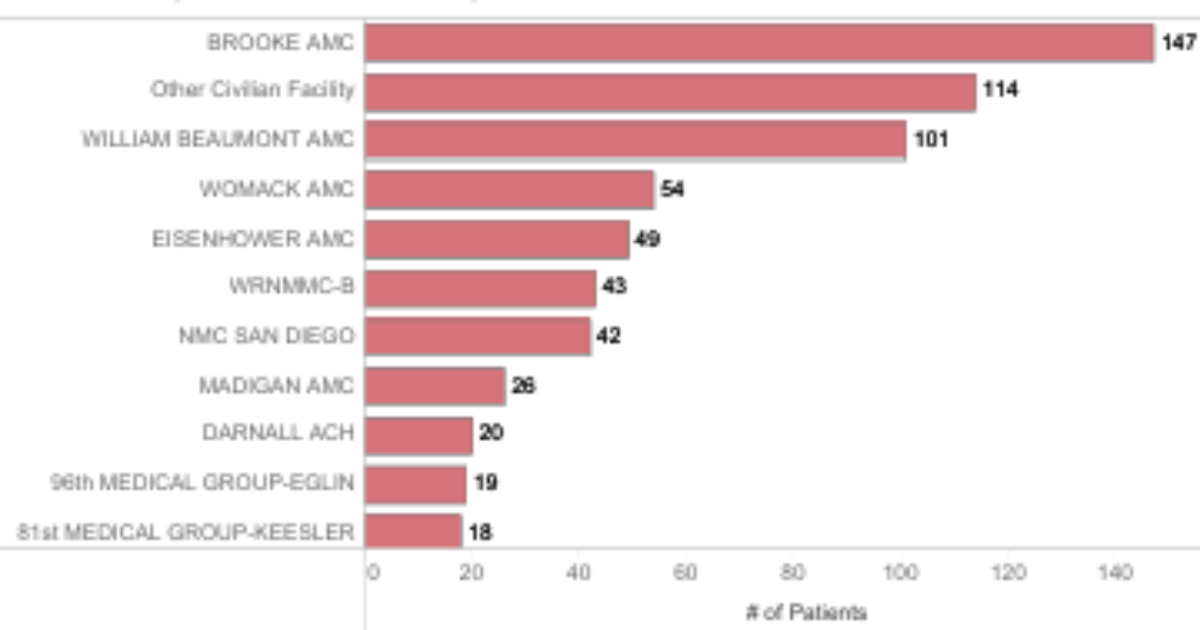
According to data provided by the whistleblower, the number of remdesivir-related deaths depicted below occurred between March 2020 and January 2023 at a variety of MTFs across the country. Eight of 11 of these locations took part in the USAMRDC trial.
Is there a causal link between the use of remdesivir and the number of deaths at these facilities or any of the others around the country? That’s a difficult question to answer because the data depicts the usage of remdesivir primarily on patients who were determined to have a severe or critical COVID-19 infection.
However, in some cases, these patients also had comorbidities (e.g., renal and liver disease) that could have been exacerbated by remdesivir and resulted in some of the reported complications such as acute renal injury and failure, as well as liver dysfunction and hepatic failure.
Obviously, not everyone who died was treated with remdesivir, but according to the whistleblower – and contrary to the results of clinical trials – “over 50 percent of those who died while hospitalized received remdesivir, [and] this was increasingly true between March 2020 and July 2021.” Coincidently, the trials listed in this report were conducted at dozens of MTFs during this same period.
This begs the following questions:
Why has the USAMRDC not published data on the number of deaths from participants in NCT04302766 nor the number of deaths of those who may not have been part of the trial itself but were treated with remdesivir in dozens of MTFs around the country?
Furthermore, is remdesivir truly effective in preserving lives – or are there alternatives with a better safety profile that should be explored? It is the “firm belief” of the whistleblower that the question presented about an alternative “was either never asked or never officially received.”
Officials from neither USAMRDC Headquarters at Fort Detrick nor any of the U.S. locations involved in NCT04302766 responded to multiple inquiries made by the author of this report. A Freedom of Information Act (FOIA) request was submitted to the USAMRDC on September 24, 2024.
Coincidentally, according to the whistleblower, the DOD’s Joint Trauma System became inaccessible on the same day the inquiries and requests were made. A week later, the site became operational.
In an effort to “retrieve insight into the clinical trial undertaken by USAMRDC,” Rep. Clay Higgins (R-LA) sent a letter on September 27 to Secretary of Defense Lloyd Austin, copying the U.S. Health and Human Services Secretary, the U.S. Food and Drug Administration Commissioner, USAMRDC Commanding General Major General Paula C. Lodi, and others.
Mis-Informed Consent
Section 6 of the Informed Consent Form for clinical trial NCT04302766 lists several common and less-common side effects attributed to remdesivir. At the time, participants were told “there have not been kidney problems seen in humans who have been given remdesivir.”
This is somewhat true, considering that in the previous Ebola virus treatment setting, there were no adverse events – to the kidneys or other organs – reported for the use of the drug. However, “the only adverse events reported in [the Ebola] trial were deaths.”
Regarding the treatment of people in Africa with the Ebola virus, the form simply read: “Some of these people had side effects.” There was no mention that the use of remdesivir resulted in the highest mortality rate among participants in a trial of four investigational therapies for Ebola in the Democratic Republic of Congo.
For this reason, it’s interesting to note that neither the Department of the Army Surgeon General nor the USAMRDC makes mention of the possibility of death in the informed consent provided to participants in the trial. Why? Were deaths during the trial inaccurately attributed to COVID-19 when they could/should have been attributed to the use of remdesivir?
It’s also interesting to note that as early as May 2020, MedPage Today was encouraging health professionals to be on the lookout for adverse event risks involving the liver and kidneys. Only the mention of a common side effect – an increase in liver function test results – was included. Nothing was shared of the possibility of poor liver function.
Four years later, it has been clearly determined that the use of remdesivir can result in many potential organ-based adverse effects. These include:

Again, were deaths during remdesivir treatment trials wrongly attributed to COVID-19 when they could/should have been attributed to the use of the controversial drug? How many participants died? Some have independently concluded that COVID-19 was not responsible for kidney issues, for example – so what did they die from?
Section 7 of the Informed Consent Form stated: “A description of this treatment protocol will be available on
http://www.ClinicalTrials.gov
, as required by U.S law.” It was also conveyed that a summary of results would be made available. To the contrary, neither has been provided.

While the other trials provided the dosage given to participants, the USAMRDC trial did not. Is this not required by U.S. law as stated in the informed consent?
Section 16 of the Informed Consent Form provided that “remdesivir treatment may be stopped, with or without your consent, if: 2) Your safety or health may be negatively affected.”
How many participants were removed from the trial due to any one of the potential organ-based adverse effects listed above?
Path to Tens of Millions of Dollars
In November 2019, the administration of then-president Donald Trump sued Gilead Sciences for “profiting billions of dollars off taxpayer research without paying royalties” and charging patients up to $20,000 a year for HIV-prevention drugs, according to The New York Times.
By spring 2020, Public Citizen estimated that taxpayers contributed at least $70.5 million for the development of remdesivir. According to The Washington Post, “Three federal health agencies were deeply involved in remdesivir’s development every step of the way, providing tens of millions of dollars of government research support.” These agencies are likely the National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), and DOD.
According to The Intercept, the FDA granted an “orphan” drug status for remdesivir, allowing Gilead to profit exclusively for seven years. Fact-checked by USA Today, “The claim that pharmaceutical company Gilead Sciences requested and received approval for a special status for a potential COVID-19 treatment is TRUE.”
The Wall Street Journal reported in June 2020 that “the government price [for remdesivir] will be $390 a dose, or $2,340 a patient for the shortest treatment course and $4,290 for a longer treatment course.” An analysis shared by Public Citizen revealed that remdesivir could have been priced at less than $1 per dose, or $10 per full treatment, and still earn a reasonable profit for Gilead.
By July 2020, Sen. Elizabeth Warren (D-MA) and a group of lawmakers began calling attention to the high price, saying, “It is profoundly unfair to the American taxpayers that furnished over $70 million in research costs for the drug to now be stuck paying the highest prices in the world.”
Despite its high cost, which resulted in an enormous financial benefit to Gilead, it’s important to note that the drug’s lethality continued to be ignored.
Transparency Matters
In June 2015, Gilead stock peaked at $115.46 per share. As remdesivir was being offered as a treatment for Ebola, Gilead stock was approximately $64-$65 per share in mid-2017, climbing to over $80 per share by fall. After failing to tackle Ebola, by the start of 2020 Gilead stock was closing at about $63-$65 per share. And by April 2020, as the U.S. announced its purchase of “virtually all” the remdesivir around the world, Gilead stock began to trend upward, closing at over $83 per share by the end of the month.
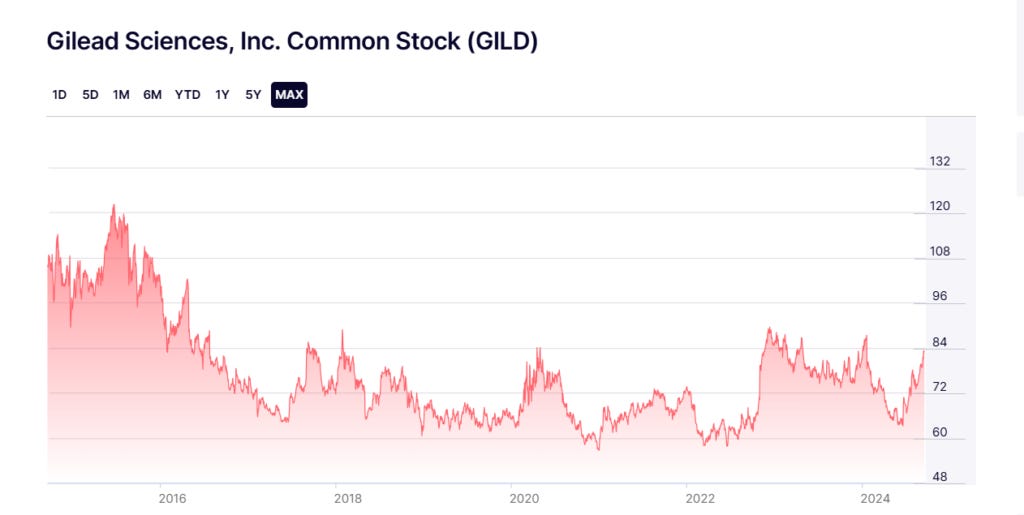
Did investors take notice of an opportunity prior to the FDA’s February 2020 approval of remdesivir to be used in clinical trials? Curiously, in February, the wife of Sen. Rand Paul (R-KY) purchased between $1,000 and $15,000 of Gilead stock.
While trade disclosures are typically reported within 45 days, Paul’s reporting did not occur until 16 months later. According to Paul spokesperson Kelsey Cooper, the initial filing was improperly transmitted, and the investment was made with the earnings of Paul’s wife.
While Paul, a medical doctor and respected senator, remains a strong voice against COVID-19 public health guidances and more, the timely investment remains suspicious. Considering the known lethality of remdesivir, it further heightens concern.
However, considering the sharp rise of millions of dollars spent by Gilead’s lobbying efforts in 2019, it’s not surprising that members of Congress and others might be more familiar with the drug manufacturer’s name in recent years.
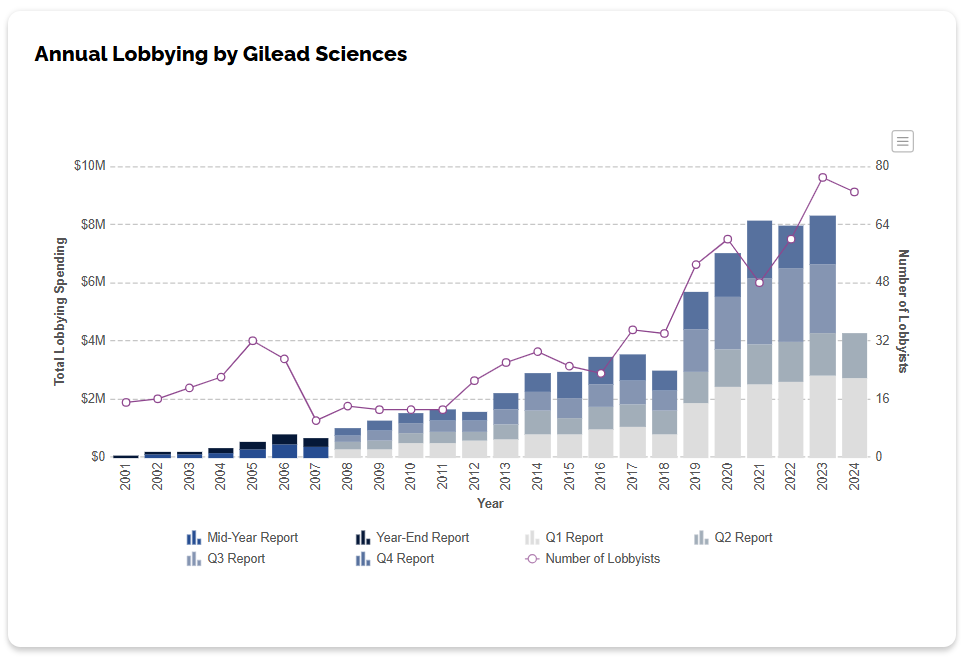
Between 2018 and 2021, total lobbying spending increased from about $3 million dollars to $8.1 million dollars – a 170-percent increase. OpenSecrets.org reveal’s the organization’s top recipients.
Specific to the military, the government transparency site also reveals the Department of Defense and Department of Veterans Affairs obligation of nearly $12 million between 2019 and 2023 to Gilead Sciences, as well as authorized Veklury (remdesivir) distributors ASD Specialty Healthcare and AmerisourceBergen. An awarding subagency of the DOD included the Defense Logistics Agency (DLA).
Deaths from remdesivir and the entire standard-of-care procedure were not limited to the five clinical trials, nor were they limited solely to military treatment facilities. The data provided in this report should warrant an investigation.
What a whistleblower has revealed should challenge military leaders and government officials to provide the truth of what happened in 2020 and beyond. Families deserve to know whether their loved ones were killed by COVID-19 or the liberal usage of remdesivir in military treatment facilities across the country and abroad.’
___
You must not wait for another catastrophic crisis (at times manufactured but we are prevented from making our own basic personal decisions or accessing needed drugs and response tools) to catch you off-guard. We must take charge and be prepared today so that we can enjoy peace of mind tomorrow.
Enter the Wellness Company as a solution and a willing participant in the health care conversation. From telemedicine, prescriptions, memberships, and supplements, TWC is leading America with alternative choices to the traditional health care model.
If you wish to give a donation to help me, you can at:
Zelle:
sr7283@gmail.com
Or Ko-Fi
Ko-fi.com/drpauleliasalexander
Or to my address at:
150 South 8th Street
Unit 170
Lewiston, New York
14092
Alternatively, please consider going from an UNPAID subscriber or follower to a PAID at $5 per month or $30 per year. This can provide me help. If this is not possible at this time, this is ok, please remain a subscriber for FREE and there is no difference between FREE and PAID. No restrictions.
Please consider support of a good company Drs. McCullough, Risch, Thorp, myself support (they are our sponsors), The Wellness Company; see the emergency preparation kit (key component being antibiotics you were denied by doctors, pharmacists, governments during the fraud COVID), first aid kit, travel emergency kit, contagion control kit etc. Please consider the SPIKE SUPPORT (spike protein DETOX dissolving spike from mRNA vaccine, this is critical to remove spike form the mRNA vaccine/and DNA viral vector) formula with NATTOKINASE as well as the triple formula (SPIKE SUPPORT, BROMELAIN, CIRCUMIN)

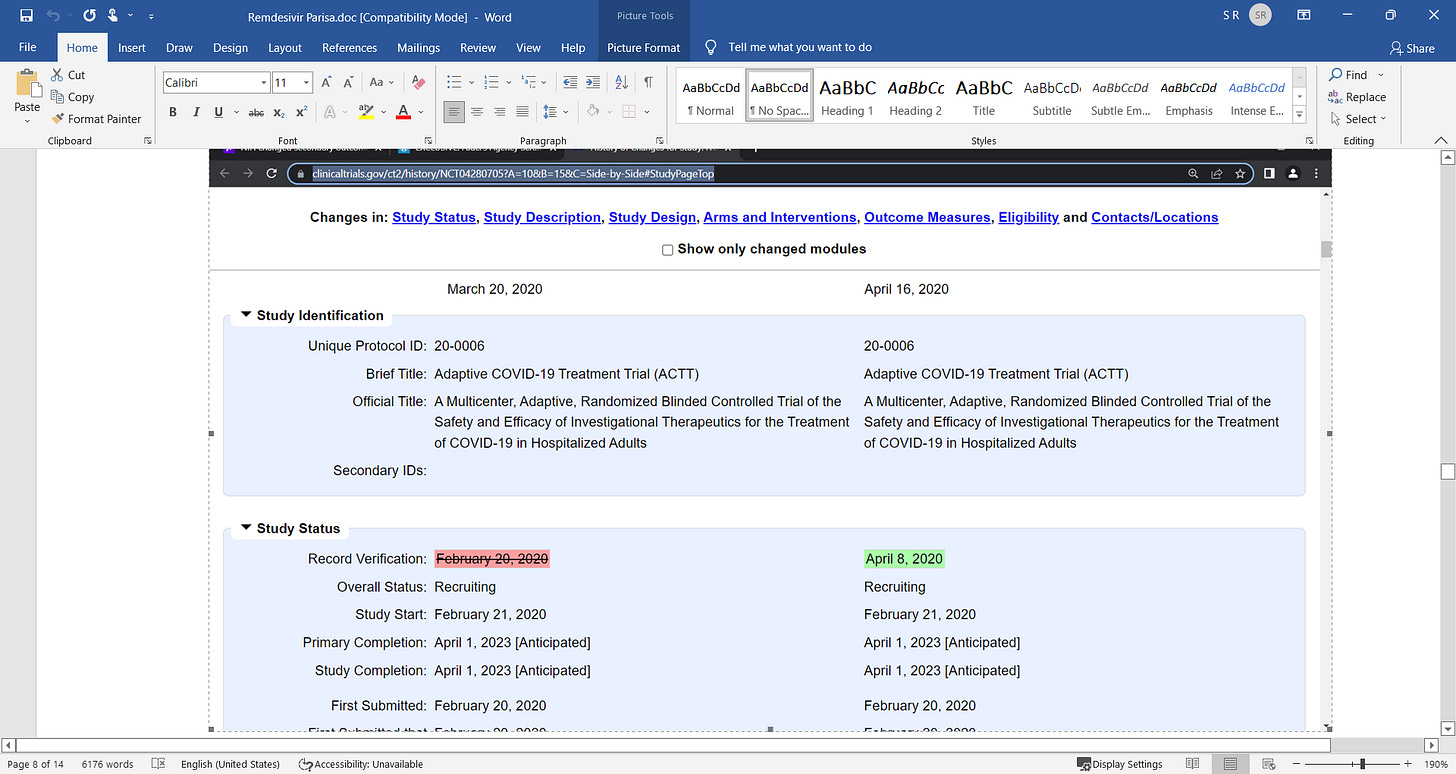
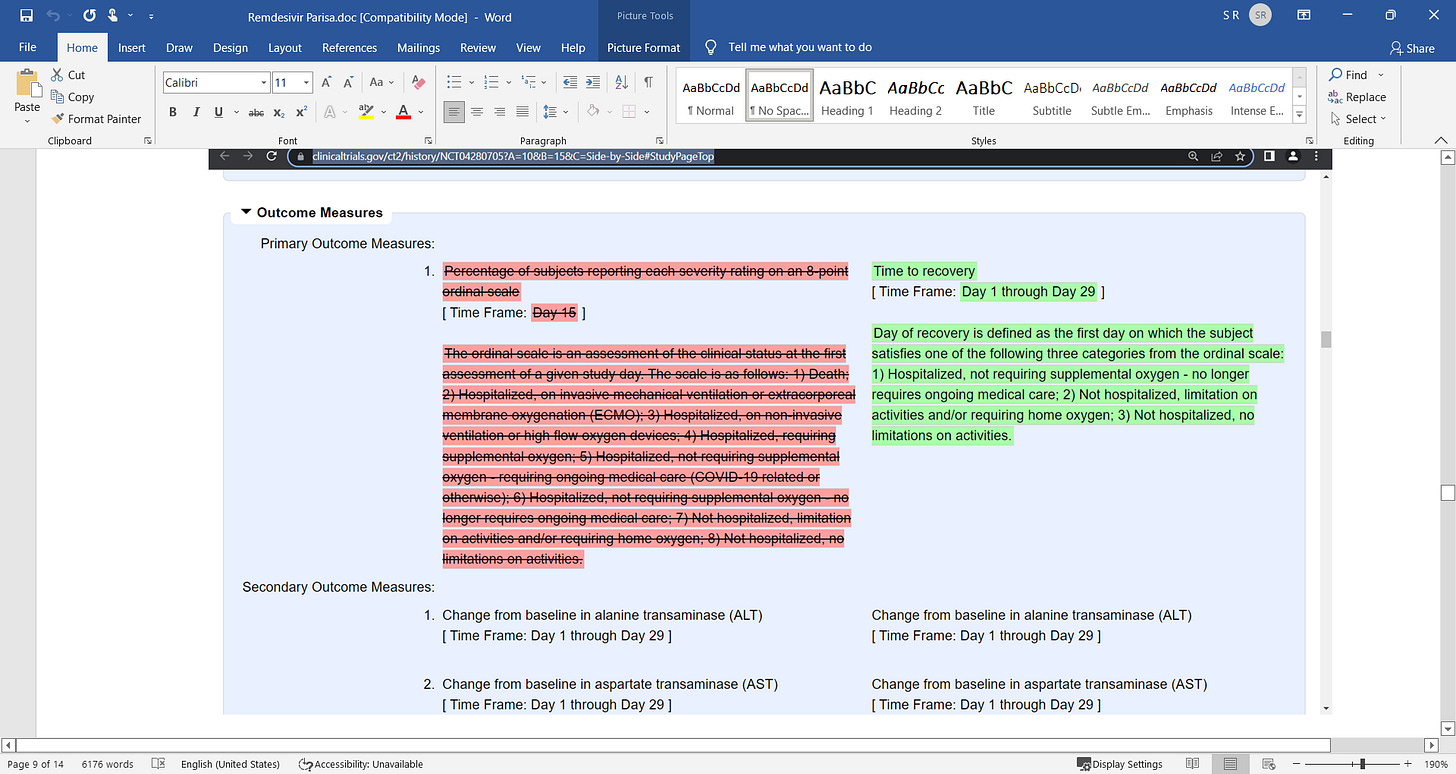
Remdesivir was part of the COVID death protocol that killed...period. a failed Kidney and liver toxic drug.
Fauci et al. know that they were defrauding the people and that Remdesvir was harmful...thats the reality