URGENT: excellent stack by McCullough! I too have ZERO interest in mRNA technology vaccine for respiratory synctial virus (RSV) like common cold; rare mild illness easily treatable, NO vaccine needed
"in the case of RSV illness is so mild & easily treatable with albuterol and budesonide nebulizers; hard to make case for mass vaccination with novel mRNA vaccine"; 1 Moderna mRNA pericarditis 42 days
See my views next but I ask to support McCullough’s work.
My view:
That one pericarditis case due to the vaccine as per McCullough should snuff out any interest, this RSV vaccine is like having a third male breast, it’s of no use, two are of no use and a third laughable…can you tell me what is the scenario to make you want a third male tit? so is this RSV vaccine, the need is not there for a rare mild cold like condition that is already well treatable…its akin to Monsanto injecting cows to produce more milk when we had enough milk and then drive mastitis in the cow’s tits and thus need for antibiotics that got into the meat…see the madness?
Yet, this RSV is ‘easily treatable with albuterol and budesonide nebulizers’. So why?
https://pubmed.ncbi.nlm.nih.gov/38091530/
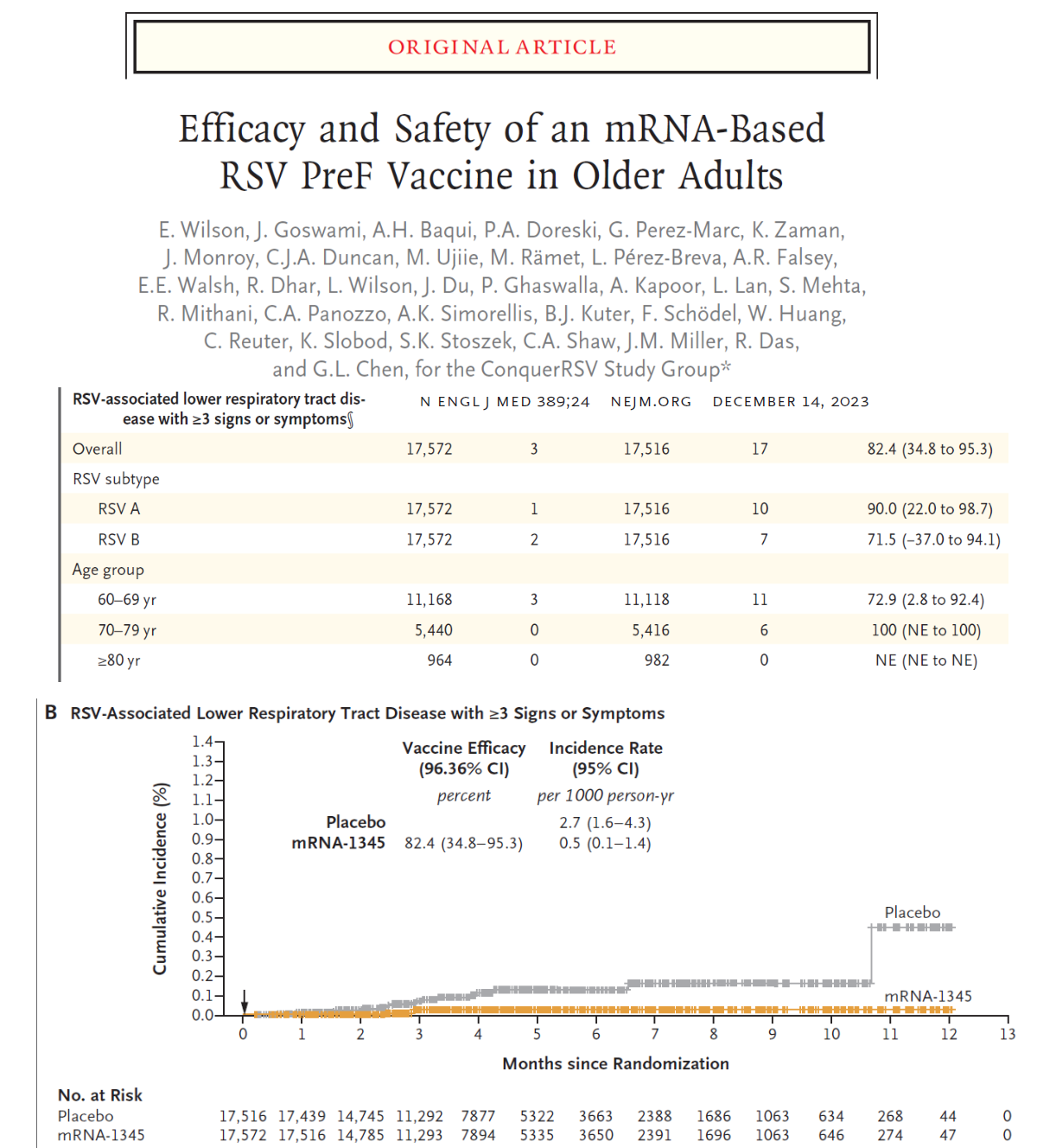
‘Vaccine efficacy was 83.7% (95.88% confidence interval [CI], 66.0 to 92.2) against RSV-associated lower respiratory tract disease with at least two signs or symptoms and 82.4% (96.36% CI, 34.8 to 95.3) against the disease with at least three signs or symptoms. Vaccine efficacy was 68.4% (95% CI, 50.9 to 79.7) against RSV-associated acute respiratory disease. Protection was observed against both RSV subtypes (A and B) and was generally consistent across subgroups defined according to age and coexisting conditions. Participants in the mRNA-1345 group had a higher incidence than those in the placebo group of solicited local adverse reactions (58.7% vs. 16.2%) and of systemic adverse reactions (47.7% vs. 32.9%); most reactions were mild to moderate in severity and were transient. Serious adverse events occurred in 2.8% of the participants in each trial group.’
‘By Peter A. McCullough, MD, MPH
Rare illnesses which are mild should not be the target for mass vaccination. Because so few people get the problem, and in the case of respiratory syncytial virus, the illness is so mild and easily treatable with albuterol and budesonide nebulizers, it is hard to make the case for mass vaccination with a novel mRNA platform.
Nevertheless, Moderna conducted a large randomized trial in adults at “high-risk” for RSV reporting only 20 cases (with three symptoms such as nasal congestion, cough, fever) out of 35,541 subjects (rate=.056%). Safety grade 3 or higher events at 28 days were reported in 4.0% of Moderna and 2.9% of placebo patients, respectively. The most common solicited systemic adverse reactions were fatigue, headache, myalgia, and arthralgia. There was one case of Moderna mRNA pericarditis at 42 days. The short-term vaccine efficacy was 82.4%.
I do not recommend adult RSV vaccination of any type since this is a very mild and rare infection in adults indistinguishable from the common cold. One serious side effect should be enough to kill interest in more clinical development.’





Dr. Paul I'm an Iraq war vet on the burn pit registry DX with asthma for which I had Albuterol and budesinide inhalers prescribed to me by the VA. You'll never guess what the VA removed from their formulary this past year.
Wait for it.............
Budesinide.
Now I wonder why they would do that? (Thick sarcasm)
Paul, come to Montreal to explain this. Uh, no, don't come to Montreal you could be stoned to express doubts about the efficacy of ANY vaccine. We're still rolling up our sleeves here. Even a pregnant woman at work, 3 weeks ago received cov and flu, now awaits whooping cough vax tomorrow.