SAGE HANA'S newsletter I highly recommend, again this one is sweeping, intense: "Self-Amplifying" mRNA COVID-19 Vaccine...AND...a "Self Assembled Nanoparticle Vaccine"
Bill Gates and Johns Hopkins told you what they were going to do back in 2018. Guess who is there as always hiding out in plain sight? I am always learning reading SAGE's, please support
‘Tokyo-based Meiji Seika Pharma received approval for manufacturing and marketing its Kostaive sa-mRNA COVID-19 vaccine, the company announced in a Nov. 28 press release. The mRNA in the vaccine is designed to self-amplify when delivered into cells, which generates a “strong immune response and the potential for extended duration of protection.” The vaccine is intended for primary immunization (2 doses) as well as booster immunization in adults. Kostaive is the "world's first approved product applying self-amplifying mRNA technology," according to the press release.
Both mRNA and sa-mRNA are RNA vaccines that use a virus’ genetic code against it. When an mRNA vaccine is injected into an individual, the mRNA instructs cells to make a specific protein and thus stimulates immune response. An sa-mRNA vaccine takes this concept further by making multiple mRNA copies, which ends up generating more spike protein.
Toby Young, general secretary of the Free Speech Union, a public interest group, pointed out in a Nov. 30 X post that the sa-mRNA vaccine was approved in Japan “despite only testing it on 800 people, no control group and only checking antibody levels not infection rates. Medicine regulation died with Covid.”
A phase 3 study compared the Kostaive ARCT-154 vaccine to Pfizer’s Comirnaty mRNA COVID-19 vaccine. The pre-print study, which has not been peer-reviewed, was posted in July at MedRxiv.
The study, funded by the Japanese Ministry of Health, Labour and Welfare, followed a primary phase study that analyzed the safety and efficacy of the Kostaive vaccine. The results of that study have not been published; the manuscript is “in preparation," according to the phase 3 study report.
The trial was conducted among 828 people between December 2022 and February 2023. This is a far lower number of participants than Pfizer’s phase 3 study, which involved over 40,000 individuals. The small scale of Kostaive trial has raised questions about its validity.
According to the pre-print study, Kostaive recipients reported a slightly lower number of localized reactions—such as localized pain or swelling—compared to Comirnaty. However, Kostaive recipients reported higher numbers in specific adverse events such as chills, diarrhea, dizziness, headache, malaise, nausea, and myalgia, or muscle pain.
According to Meiji Seika Pharma, the phase 3 clinical trials for booster shots showed that Konstaive elicited “higher and longer-lasting neutralizing antibody titers against the original strain” as well as an Omicron subvariant, compared to Comirnaty.
The vaccine was developed by San Diego-based Arcturus Therapeutics. Meiji Seika Pharma licensed the vaccine for sale in Japan via Melbourne-based CSL Seqirus in April this year.
The company is collaborating with Arcalis, an mRNA vaccine manufacturing firm, to establish manufacturing capabilities in Japan. Meiji Seika Pharma is working towards commercializing Kostaive in 2024.
Risks of sa-mRNA
As sa-mRNA vaccines produce copies of mRNA and thus boost the production of proteins, some experts are worried about the consequences they can have on the human body and concerned that any negative effects from mRNA vaccines could be amplified by injecting sa-mRNA shots.
During testimony at the European Parliament last month, cardiologist Peter McCullough said that “there's not a single study showing that the messenger RNA is broken down” in the human body once it is injected. Since the vaccines are “made synthetically, they cannot be broken down.”
The spike protein from the mRNA vaccines has been found circulating in the body as long as six months from vaccination, he pointed out.
Dr. McCullough said that the spike protein is “proven” in 3,400 peer-reviewed manuscripts to cause four major domains of disease—cardiovascular, neurological disease, blood clots, and immunological abnormalities.
In a recent Epoch Times article, molecular biologist Klaus Steger noted that “a small amount of saRNA [sa-mRNA] results in an increased amount of produced antigen.”
“Due to increased antigen levels, one injection of saRNA—whether linear or circular—may cause adverse events comparable with repeated (booster) injections of modRNA.”
Mr. Steger had previously pointed out that BioNTech's "mRNA" vaccines are made not with messenger RNA but with modified RNA (modRNA).
A study published in the journal Trends in Biotechnology in June this year admitted that the “main challenges involved in the global authorization [of sa-mRNA vaccines] are potential safety concerns regarding the replicative character of these vaccines.”
July 13, 2022, Korea
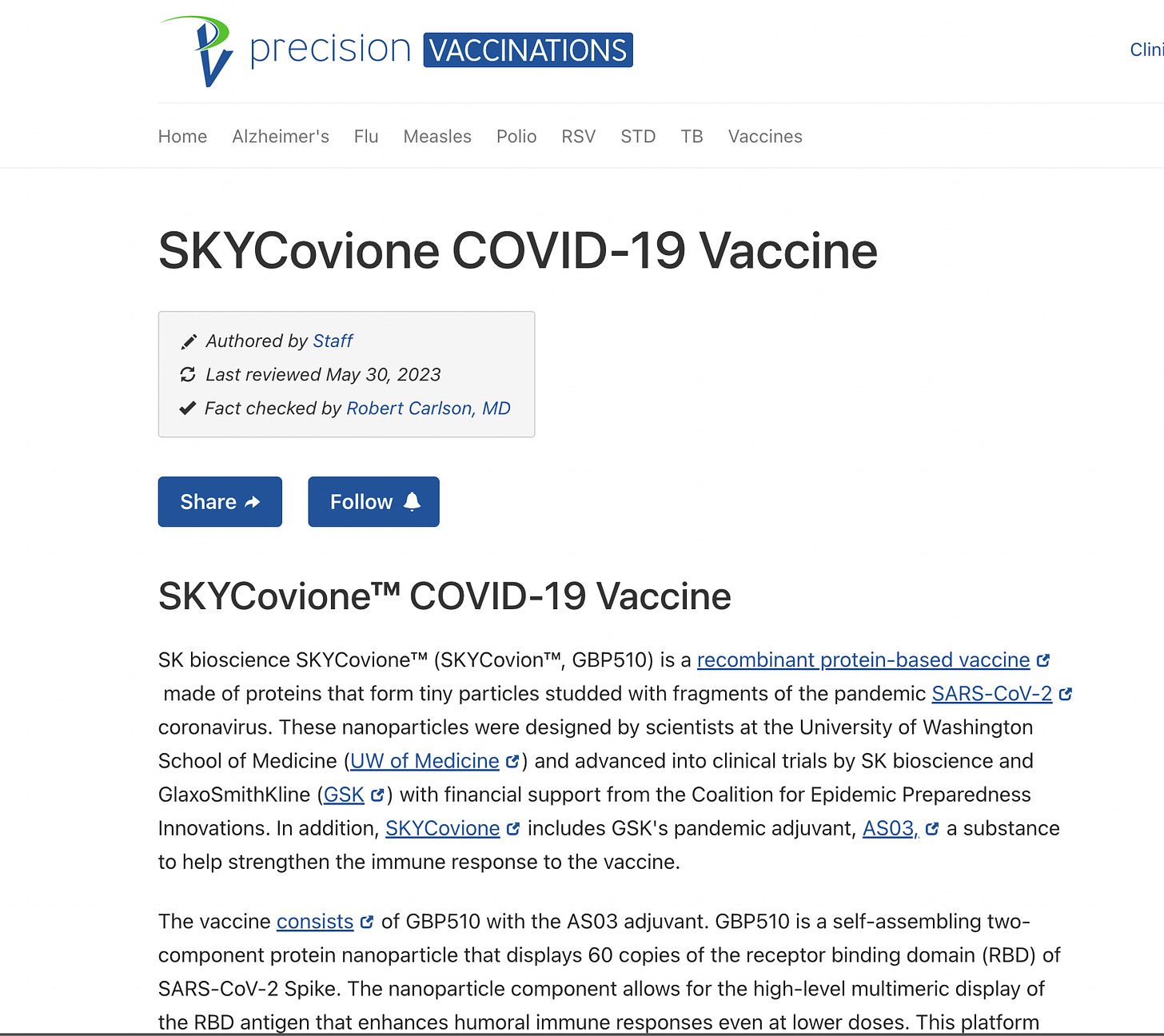
SKYCovione(TM) is a self-assembled nanoparticle vaccine
editor’s note: Where have I heard this description before?
SKYCovione(TM) is a self-assembled nanoparticle vaccine targeting the receptor binding domain of the SARS-CoV-2 Spike protein for the parental SARS-Cov-2, jointly developed with the Institute for Protein Design (IPD) at the University of Washington School of Medicine with combination of GSK's pandemic adjuvant. The development of SKYCovione(TM) has been supported by funding from the Bill & Melinda Gates Foundation and Coalition for Epidemic Preparedness Innovations (CEPI).
May 26, 2023, UK
SKYCovion, the COVID-19 vaccine developed by SK Chemicals, has today been authorised by the Medicines and Healthcare products Regulatory Agency (MHRA).
It becomes the 8th COVID-19 vaccine authorised by the UK’s independent medicines regulator.
The authorisation follows advice received from the independent Commission on Human Medicines (CHM).
The SKYCovion vaccine combines a part of the SARS-CoV-2 virus spike protein with an ‘adjuvant’ – an additional ingredient designed to trigger a stronger immune response. It is given as two injections, four weeks part.
The vaccine consists of GBP510 with the AS03 adjuvant. GBP510 is a self-assembling two-component protein nanoparticle that displays 60 copies of the receptor binding domain (RBD) of SARS-CoV-2 Spike. The nanoparticle component allows for the high-level multimeric display of the RBD antigen that enhances humoral immune responses even at lower doses. This platform also allows for simple and efficient adjustment of the vaccine to match variant strains of SARS-CoV-2 or other emergent pathogens by swapping out the target antigen, which the nanoparticle displays. In addition, the inclusion of AS03 may allow for the use of lower doses of protein antigen.
Back to the 2022 Korean Approval literature.
The Korean Ministry approved SKYCovione of Food and Drug Safety for use on June 29, 2022, in individuals 18 years and older. In addition, the South Korean government has agreed to purchase 10 million doses for domestic use. On May 30, 2023, the UK's Medicines and Healthcare products Regulatory Agency (MHRA) granted Marketing Authorization for SK bioscience's COVID-19 vaccine SKYCovion™ as a primary series for strong immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older. On June 19, 2023, SkyCovione received Emergency Use Listing (EUL) from the World Health Organization (WHO). SKYCovione is the 12th COVID-19 vaccine granted a EUL by the WHO.
SKYCovion is the world's first-ever vaccine developed using the RoseTTAFold, a software tool that uses deep learning to quickly and accurately predict protein structures based on limited information. The RoseTTAFold was designed as a three-track neural network developed by the University of Washington.
SK bioscience is an innovative biopharmaceutical company, standing committed to global pandemic preparedness in vaccine development and manufacturing to create more equitable access to vaccines.
“a software tool that uses deep learning to quickly and accurately predict protein structures based on limited information.”
Sounds an awful lot like…
March 25, 2019
About Novotech - https://novotech-cro.com/welcome
Headquartered in Sydney, Novotech is internationally recognized as the leading regional full-service contract research organization (CRO). With a focus on clinical monitoring, Novotech has been instrumental in the success of hundreds of Phase I - IV clinical trials in the Asia Pacific region.
The Asia Pacific region…
You don’t say.
Seems like a company like that would need a good international vaccine broker.
2021 CV:
Dr. Malone has a history of assembling and managing expert teams that focus on solving complicated biodefense challenges to meet US Government requirements. He was instrumental in enabling the PHAC/rVSV ZEBOV (“Merck Ebola”) vaccine to move forward quickly towards BLA and (now recently granted) licensure.
Dr. Malone got the project on track in support of DoD/DTRA and NewLink Genetics, recruited organizations to team with USAMRIID/WRAIR to develop the immunoassays, put WHO and Norwegian government philanthropic leadership in touch with Pentagon leadership to expedite the initial WRAIR clinical and *ring vaccination trials, recruited a management team, recruited Merck vaccines to purchase the product candidate from NewLink, helped write and edit the clinical trials developed by the World Health Organization and lead the development of the BARDA and DTRA contracts - yielding over 200M$ in resources.
Dr. Malone’s early involvement in this project allowed for the Merck vaccine to be developed very rapidly.
*ring vaccination trials
Notorious GVB Warned that the Ebola Vaccine Ring Trials were Killing People: "We just wanted to have the case fatality rate of the whole period."
·
MAR 20
Good broker hired!
March 25, 2019
Dr Malone brings more than 20 years of management and leadership experience in academia, pharmaceuticals and biotechnology, with deep expertise in regulatory and medical strategy for global clinical product development. Dr Malone is an internationally recognised physician and scientist for his work in clinical trials in the areas of vaccines, gene therapy, biodefense and immunology, and specifically as one of the original inventors of DNA vaccination and multiple non-viral gene therapy technologies (RNA and DNA).
Commenting on the new role, Robert said: "I am delighted to join the CNS BioDesk USA consulting team; this is an exciting opportunity to work within a highly professional and experienced group that is dedicated to adding value to clients' early phase programs and their US regulatory strategies."
Russell Neal, CNS Managing Director said: "Dr Malone brings a wealth of industry and consulting experience to our US BioDesk team, and his appointment reflects CNS' ongoing commitment to the continuous growth and evolution of BioDesk to service CNS' international clients as they navigate US and global regulatory strategies."
CNS' BioDesk division is an intelligent global product development and regulatory affairs consultancy specialising in readying products to swiftly enter the clinic or gain marketing approval. It is a uniquely internationally experienced team with particular expertise in the areas of vaccines, oncology, infectious disease as well as cell and gene therapies.
Well, at the very least I hope that Bob set Novotech and SK Bioscience straight on vaccines for coronaviruses.
Maybe performed a threat assessment for the products that SK Bioscience was working on and developing…you know in March, 2019.
Do We Need a Vaccine for This Coronavirus? Did We Ever?
Malone:
I performed a threat assessment during the first week of January, 2020 concerning what I was able to discover regarding SARS-CoV-2, and determined that there was insufficient time to develop a safe and effective vaccine for the virus (and also noted the horrible history of prior attempts to develop human vaccines for other coronaviruses), and then spun up a highly skilled team (and got them funded) focusing on repurposing existing licensed drugs for treating the disease.
Here we quickly establish that Dr. Robert Malone is fully aware that coronaviruses do not and have not responded well to vaccine development.
When David Martin shouts about BIOLOGICAL AND CHEMICAL WARFARE at the Brussels CHD We Did It Conference…about pushing vaccines that don’t work…who is the “they”?
“You know why they didn’t work?”

Martin continues.
…It turns out that coronavirus is a very malleable model. It transforms, and it changes, and it mutates over time, and as a matter of fact, every publication on vaccines for coronavirus from 1990 until 2018…every single publication concluded that coronavirus escapes the vaccine impulse, because it modifies and mutates too quickly for vaccines to be effective.
And since 1990 to 2018, that is the published science, ladies and gentlemen. That’s following the science. Following the science is their own indictment of their own programs that said it doesn’t work.
But enough about Bob Malone!
Let’s talk about Self Amplifying Vaccines.
2018
Here was the masthead on the Johns Hopkins report.
I’ll link to a whole show that Ryan Cristian did on the topic.
In March, 2022, I wrote the following and please note that the Johns Hopkins link is now broken.
Pinned this in the comments, but this is worthy of adding to the body of the post:
Everybody, here is the direct link to the Johns Hopkins PDF:
https://jhsphcenterforhealthsecurity.s3.amazonaws.com/181009-gcbr-tech-report.pdf
Now consider this nightmare fuel paragraph contextualized with what has happened over the last two years:
"Self-Spreading Vaccines: Self- spreading vaccines are genetically engineered to move through populations like communicable diseases, but rather than causing disease, they confer protection. The vision is that a small number of individuals in a target population could be vaccinated, and the vaccine strain would then circulate in the population much like a pathogenic virus, resulting in rapid, widespread immunity."
But wait...It gets better!
"Self-Amplifying mRNA Vaccines:
SAM vaccines use the genome of a modified virus with positive sense RNA, which is recognizable to our human translational machinery. Once delivered inside a human cell, the SAM is translated and creates 2 proteins: an antigen of interest to stimulate an immune response, and a viral replicase for intracellular amplification of the vaccine. *The ability of SAM to self-replicate results in a stronger, broader, and more effective humoral and cellular immune response than some other vaccines.
Drone Delivery to Remote Locations: Drone transportation networks can enable the rapid delivery of clinical materiel and..."
*That part sure didn’t happen, Johns Hopkins Evil Geniuses!
And blah, blah, blah...Get ready for your drone delivered self amplifying "vaccines" people.
Ryan Cristian of TLAV covered this back in May, 2021. Two years ago.
His Johns Hopkins Self Spreading Vaccine link is now broken too.
It’s the sixth one down.
It now redirects to this fluffy home page.
Someone with some Internet Fu please try to locate that 2018 study, PDF or otherwise.
Nexus Newsfeed.com
Johns Hopkins links are redirecting.
The second link is the same link that Ryan used.
I don’t see a search engine on Bioweapons Hopkins Central Command and I cannot seem to find the Self Spreading Vaccine write up.
Back to Nexus Newsfeed:
Johns Hopkins on self-spreading, self-propagating, transmissible vaccines
In 2018, The Johns Hopkins Bloomberg School of Public Health, Center for Health Security, published Technologies to Address Global Catastrophic Biological Risks.
Among the technologies discussed in the paper, are "Self-Spreading Vaccines." Vaccines that only need be given to a portion of the population, and are then become communicable between individuals, like a virus.
The number of questions, concerns, and issues that become relevant with the revelation that this technology exists, and is being used on animals, is more vast than I can enumerate here. So I will simply share with you Page 47 of the document.
"WHAT IS THE TECHNOLOGY?
Self-spreading vaccines—also known as transmissible or self-propagating vaccines—are genetically engineered to move through populations in the same way as communicable diseases, but rather than causing disease, they confer protection. The vision is that a small number of individuals in the target population could be vaccinated, and the vaccine strain would then circulate in the population much like a pathogenic virus. These vaccines could dramatically increase vaccine coverage in human or animal populations without requiring each individual to be inoculated. This technology is currently aimed primarily at animal populations. Because most infectious diseases are zoonotic, controlling disease in animal populations would also reduce the risk to humans.
There are 2 main types of self-spreading vaccines: recombinant vector vaccines and live viral vaccines. Recombinant vector vaccines combine the elements of a pathogenic virus that induce immunity (removing the portion that causes disease) with a transmissible viral vector. Cyto-megalovirus is one candidate vector for recombinant vaccines, because it is highly species-specific and moderately transmissible. Live viral vaccines are attenuated, meaning that the vaccine viruses are much less pathogenic than wild-type and would be similar to the oral polio vaccine or the live attenuated influenza vaccine (LAIV) in that those vaccines can sometimes transmit from person to person.
Although there are substantial technical challenges in genetically engineering viruses, synthetic biology tools such as CRISPR/Cas9 are likely to aid researchers in overcoming these hurdles in the coming years. Self-spreading vaccines have already been used to protect wild rabbits from myxomatosis and to control Sin Nombre virus in rodent populations.
Additional work is targeting Ebola virus in apes and bats, Lassa virus in rats, and bovine tuberculosis in badgers.
WHAT PROBLEM DOES THIS SOLVE?
The most practical and useful application of self-spreading vaccines would be to control disease spread in wild animal populations (also known as sylvatic spread). A vaccine would be administered to a few selected animals in hotspots among target populations including nonhuman primates, bats, or rodents. The vaccine would then spread within the target population , eliminating the need to vaccinate each animal. Successful disease control in animal populations could limit the number of infected animals and thereby reduce the opportunity for the disease to spill over into humans, thus stopping outbreaks in humans before they ever emerge. Such a sylvatic strategy would reduce the overall number of outbreak opportunities in humans, but it could not interrupt an outbreak once it becomes established in humans.
In the event of a grave public health threat, self-spreading vaccines could potentially be used to broadly inoculate human populations. Like the approach in animals, only a small number of vaccinated individuals would be required in order to confer protection to a larger susceptible population, thus eliminating the need for mass vaccination operations, including PODs." [PODs are doctors’ offices, pharmacies, and points of dispensing.]
By Ginger Taylor, MS
Ginger Taylor, MS is an advocate at HealthChoice.org and Director of the Maine Coalition for Vaccine Choice.
(Source: ageofautism.com; May 2, 2021; https://bit.ly/3tcvjJp)
Anyway, if you can find that Johns Hopkins PDF, download it before the past is alterable.
Obviously, there are copies of the Johns Hopkins Report downloaded out there.
It may just be a matter of giving intrepid detectives another hoop to jump through as they try to track back the plans and designs.
I’ve got SPARS downloaded, and I’ve got 🐴 and Annie DeGroot in 2013 downloaded mining “emerging pathogens and biodefense” territory.
https://www.tandfonline.com/doi/full/10.4161/hv.25611
Same Op. Same Op.
It’s all honorable until the other end of that “dual use” is employed against the “terrorists”, i.e. you, hapless Human Lab Rat who objects to being part of the “live fire” test.
Don’t worry, this is just how they talk.
The US Pentagon DTRA blah blah blah live fire test, we test on the troops with the Anthrax and we test on you and your kids and it’s a live fire example and it’s only because we care so deeply.
“It is clear that a single unwanted introduction of a GM [genetically modified viral] biocontrol agent could have serious consequences. Once a persisting transmissible GMO is released (whether intentionally, legally, or otherwise), it is unlikely that it could be completely removed from the environment. The scientific community involved in developing GM biocontrols therefore needs to demonstrate a highly precautionary attitude. Scientists also have an ethical responsibility to consider the full implications of the solutions they are researching: they must be seen to be acting openly, collaboratively and responsibly” (6, p. 583).
I’m sure that Tier Two Media will be all over this.
Tier 1. Still going strong.
Tier 2.
It is even more tempting to take a negative view on all new technology when the product launch in humans fails to a large degree.
















Non-compliance Step 1:
STOP calling Vaccines technologies, which elicit an immune response.
Pathogens too result in an immune response. Should we call them vaccines?
I have a better idea, let’s call mRNA vaxx PATHOGENS. This is what they are
Untested? There was a massive clinical trial for mRNA agents. It was called the Covid Pandemic of 2020. https://twitter.com/ChildrensHD/status/1733940175687082090