
Ka-BOOM! Study (Brouqui) shows excellent results when using Azithromycin & Hydroxychloroquine as early treatment on patient-important outcomes! Not optimally a comparative effectiveness
randomized controlled trial adjusting for selection bias, known & unknown distorting confounding variables yet McCullough's stack handles this well as we don't need that with these potent results
https://www.sciencedirect.com/science/article/pii/S2052297523001075
‘Results
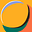
Among 30,202 patients for whom information on treatment was available, 191/23,172 (0.82%) patients treated with HCQ-AZ died, compared to 344/7030 (4.89%) who did not receive treatment with HCQ-AZ. HCQ-AZ therapy was associated with a lower mortality than treatment without HCQ-AZ (odds ratio (OR) 0.16; 95% confidence interval (CI), 0.14–0.19). After adjustment for sex, age, period, and patient management, HCQ-AZ was associated with a significantly lower mortality rate (adjusted OR (aOR) 0.55, 95% CI 0.45–0.68). On a subsample of 21,664 patients with available variant information, results remained robust after adjustment on sex, age, patient management and variant (aOR 0.55; 95% CI 0.44–0.69). On a subsample of 16,063 patients, HCQ-AZ was still associated with a significantly lower mortality rate (aOR 0.47, 95%CI 0.29–0.75) after adjustment for sex, age, period, patient management, vaccination status and comorbidities.
Conclusion
Analysis of this large online database showed that HCQ-AZ was consistently associated with the lowest mortality.’
When I look at the research methods, it was strong enough for me to be comfortable with the methodology, e.g.:
Researchers performed both ‘univariate and multivariable logistic regressions with death as the outcome’ (patient-important outcome).
‘Multivariate logistic regressions were adjusted for sex, age groups (<50, 50–69, 70–89 and > 89 years), periods (or variants), and type of patient management (inpatient/outpatient).’
Researchers ‘performed stratified multivariable logistic regressions according to these covariates. Given that the French National Death Registry is near exhaustive, researchers decided that there were no missing data regarding outcomes. This is a reasonable assumption.
‘No data was missing regarding sex or period of admission. Treatment data were missing for a total of 221 patients. Since the proportion of patients with missing treatment data was very low (0.7%), they were excluded from the univariate and multivariable analyses of associations between treatment and death. Information on a total of 14,360 patients (47.2%) was missing regarding their vaccination status and comorbidities, and information on SARS-CoV-2 variant was missing or unknown for 8,759 patients (28.8%). Comorbidities, vaccinations, and variants were used as covariates in different subgroup analyses.’
McCullough’s opinion in stack:
‘We perform prospective, randomized, double-blind, placebo-controlled trials to test drugs, vaccines, devices, and other products for safety and efficacy. Randomization is important since it handles: 1) selection bias, 2) all known and unknown confounders. Despite the hundreds of billions of dollars spent during the pandemic, we did not have an investment in large, multidrug prospective, randomized, placebo controlled trials or comparative studies to test the best drug regimens.
In the end, what patients care about is how they feel, function, and survive. When it came to COVID-19, whether randomized or not, if patients survived if they were in the optimally treated group. The only way to assess how a high-risk population faired in the pandemic is to report on a large sample of patients sick with COVID-19 with a large number of the outcome of of interest—death.
Brouqui et al reported from a French database of 30,423 COVID-19 patients of whom 535 succumbed to the illness. In great detail, the investigators report mortality according to ambulatory treatment received, hospitalization, and the course over the following six weeks.
As you can see, the most favored group was those who received the regimen of hydroxychloroquine and azithromycin early in the course of illness. Of the 30,202 patients for whom treatment information was available, 191/23,172 patients (0.82%) treated with HCQ-AZM died, compared to 344/7,030 patients (4.89%) who did not receive HCQ-AZM. All the other combinations received are reported in the figure.
Important points:
HCQ+AZM consistently reduced the risk of hospitalization and death
If hospitalized, those pre-treated with HCQ+AZM at home had a greater chance of survival
Critics say this was not a randomized trial. Patients say it does not matter, they just want to survive on HCQ + AZM! When the differences are this large, we go with what is working for patients, not a false narrative from the Bio-Pharmaceutical Complex deceiving the population on simple, safe, generic drugs.’








Basically, everything we were told wouldn't work against COVID initially does...
All the drugs that work are anti-parasite drugs and OTC supplements.
Something stinks.