
Notorious GVB (Vanden Bossche) warned that the Ebola Vaccine Ring Trials were Killing People: "We just wanted to have the case fatality rate of the whole period." Geert got told to STFU (Sage Hana)
Sage Hana's substack on Ebola Ring trials is eye-opening and does a nice job highlighting Geert; Sage's views are quite interesting & in the interest of no censor, I share
‘
Background: Geert’s Lonely Piano
https://thehighwire.com/videos/vaccine-expert-warns-of-covid-vaccination-catastrophe/
I want to try out smashing one salient point and not get too far in the weeds. The following is a tightened up post that I did in October.
Watch from 6:00 to about 13:30.
Seven minutes of your life.
Notorious GVB describes the problems with the 2014-2016 Ebola Ring Trial. Del Bigtree and staff do a great job of translating. It is understandable.
In advance, I’m way above my paygrade here on the the science, and on the Private Public partnerships.
So feel free STEMs and Industry Vets to guide me if I’m going astray.
Did you watch the seven minutes of Geert on Del?
So far so good?
A study was designed poorly and Geert sounded the alarm and got told to sit down by the WHO, et. al.
This Ebola trial appears to be mentioned here by Dr. Robert Malone.
I am trying very hard not to wade into “dark conspiracy”, and adhere exactly to documentable evidence, such as a copy and paste from Malone’s 2021 CV.
Dr. Malone has a history of assembling and managing expert teams that focus on solving complicated biodefense challenges to meet US Government requirements. He was instrumental in enabling the PHAC/rVSV ZEBOV (“Merck Ebola”) vaccine to move forward quickly towards BLA and (now recently granted) licensure. Dr. Malone got the project on track in support of DoD/DTRA and NewLink Genetics, recruited organizations to team with USAMRIID/WRAIR to develop the immunoassays, put WHO and Norwegian government philanthropic leadership in touch with Pentagon leadership to expedite the initial WRAIR clinical and ring vaccination trials, recruited a management team, recruited Merck vaccines to purchase the product candidate from NewLink, helped write and edit the clinical trials developed by the World Health Organization and lead the development of the BARDA and DTRA contracts - yielding over 200M$ in resources. Dr. Malone’s early involvement in this project allowed for the Merck vaccine to be developed very rapidly.
Here is a Housatonic with a short video with some more background.
Spotting a problem with the Vaccine Science and sounding the alarm and getting told to Sit Down and STFU did not begin with COVID-19 for Geert Vanden Bossche.
Notorious had a warm up beatdown in trying to slow the Global Public-Private Partnership Vaccine train barreling down the tracks with the Ebola Trials, whilst working with GAVI.
That warning was ignored, too.
Once this train starts rolling, and once the WHO opens the door to industry, you know what you’re going to get. Those guys are not concerned or worrying about our health. This is about, of course, the shareholders; enriching the shareholders.
GVB, CHD Friday Roundtable, August 26, 2022
helped write and edit the clinical trials developed by the World Health Organization and lead the development of the BARDA and DTRA contracts - yielding over 200M$ in resources.
Dr. Robert Malone
He said it. I didn’t.
So what happened? Can I ask that and not get sued?
WTF happened?
Early Background of the Ebola Vaccine Campaign, 2014
ClinicalTrials.gov, a global registry of trials involving human subjects, lists several Ebola vaccine trials in progress. Ebola Zaire is the strain of the virus that is responsible for the 2014 outbreak. Therefore, all the vaccine candidates being advanced are designed to prevent that strain. If these vaccines work for Ebola Zaire, it is likely that the same principles can be applied to the other strains.
The two front-running vaccine candidates are a GSK chimpanzee adenovirus vector vaccine (including several versions) and a Merck/NewLink Genetics recombinant vaccine. Both are being tested in a single Phase 2 trial in Liberia in those at risk for EVD. The trial is being run by NIAID/NIH and began recruiting participants in fall 2015.
The Ebola vaccine licensed by NewLink Genetics in Ames, Iowa, was originally developed by the Public Health Agency of Canada, which still holds intellectual property rights for it. The vector for this monovalent Ebola Zaire vaccine is an attenuated vesicular stomatitis virus -- a virus, like rabies virus, in the Rhabdoviridae family. Vesicular stomatitis virus (VSV) can infect humans, though this is a self-limited infection. A safe VSV vaccine for animals has been developed for animal use, but it is not currently marketed in the United States.
The version of the GSK Ebola vaccine in the Phase 2 trial is monovalent and offers protection from Ebola Zaire only. This vaccine uses an adenovirus to deliver key Ebola antigens to human cells. Adenoviruses can cause various diseases, but attenuated adenoviruses are safe and have been studied as vaccine vectors. A related bivalent (Ebola Zaire and Ebola Sudan) chimpanzee adenovirus vaccine is being tested in a Phase 1 trial at the NIH Clinical Center.
https://historyofvaccines.org/diseases/ebola-virus-disease
The Ring Ebola Vaccination Trials that Notorious GVB is Discussing
Abstract
The 2014-2016 Ebola outbreak caused over 28,000 cases and 11,000 deaths. Merck & Co. Inc., Kenilworth, NJ USA and NewLink Genetics are working with private and public partners to develop and license an Ebola vaccine that was evaluated extensively during the outbreak. The vaccine referred to as V920 is a recombinant vesicular stomatitis virus (rVSV) in which the VSV-G envelope glycoprotein (GP) is completely replaced by the Zaire ebolavirus GP (rVSVΔG-ZEBOV-GP). Eight Phase I and four Phase II/III clinical trials enrolling approximately 17,000 subjects were conducted in parallel to the outbreak to assess the safety, immunogenicity, and/or efficacy of V920. Immunogenicity data demonstrate that anti-GP antibodies are generally detectable by ELISA by 14days postvaccination with up to 100% seroconversion observed by 28days post dose. In addition, the results of a ring vaccination trial conducted by the WHO and their partners in Guinea suggest robust vaccine efficacy within 10days of receipt of a single dose of vaccine. The vaccine is generally well-tolerated when administered to healthy, non-pregnant adults. The development of this vaccine candidate in the context of this unprecedented epidemic has involved the close cooperation of large number of international partners and highlights what we as a public health community can accomplish when working together towards a common goal. Study identification: V920-001 to V920-012. CLINICALTRIALS.GOV identifiers: NCT02269423; NCT02280408; NCT02374385; NCT02314923; NCT02287480; NCT02283099; NCT02296983; NCT02344407; NCT02378753; NCT02503202.
Keywords: Clinical trials; Ebola vaccine; Efficacy; Immunogenicity; Recombinant; Safety.
Copyright © 2017. Published by Elsevier Ltd.
Funding
WHO, UK Wellcome Trust, the UK Government through the Department of International Development, Médecins Sans Frontières, Norwegian Ministry of Foreign Affairs (through the Research Council of Norway's GLOBVAC programme), and the Canadian Government (through the Public Health Agency of Canada, Canadian Institutes of Health Research, International Development Research Centre and Department of Foreign Affairs, Trade and Development).
And Dr. Malone acted as Vaccine Sherpa pulling together the public-private partnership, per his CV.
He’s a regulatory expert.
The Problem with the Trials According to Geert
Geert openly discusses the issues that he had with the New Link Genetics/Merck Ebola rVSV-ZEBOV Vaccine Trials in Guinea, Africa in the video which you just watched.
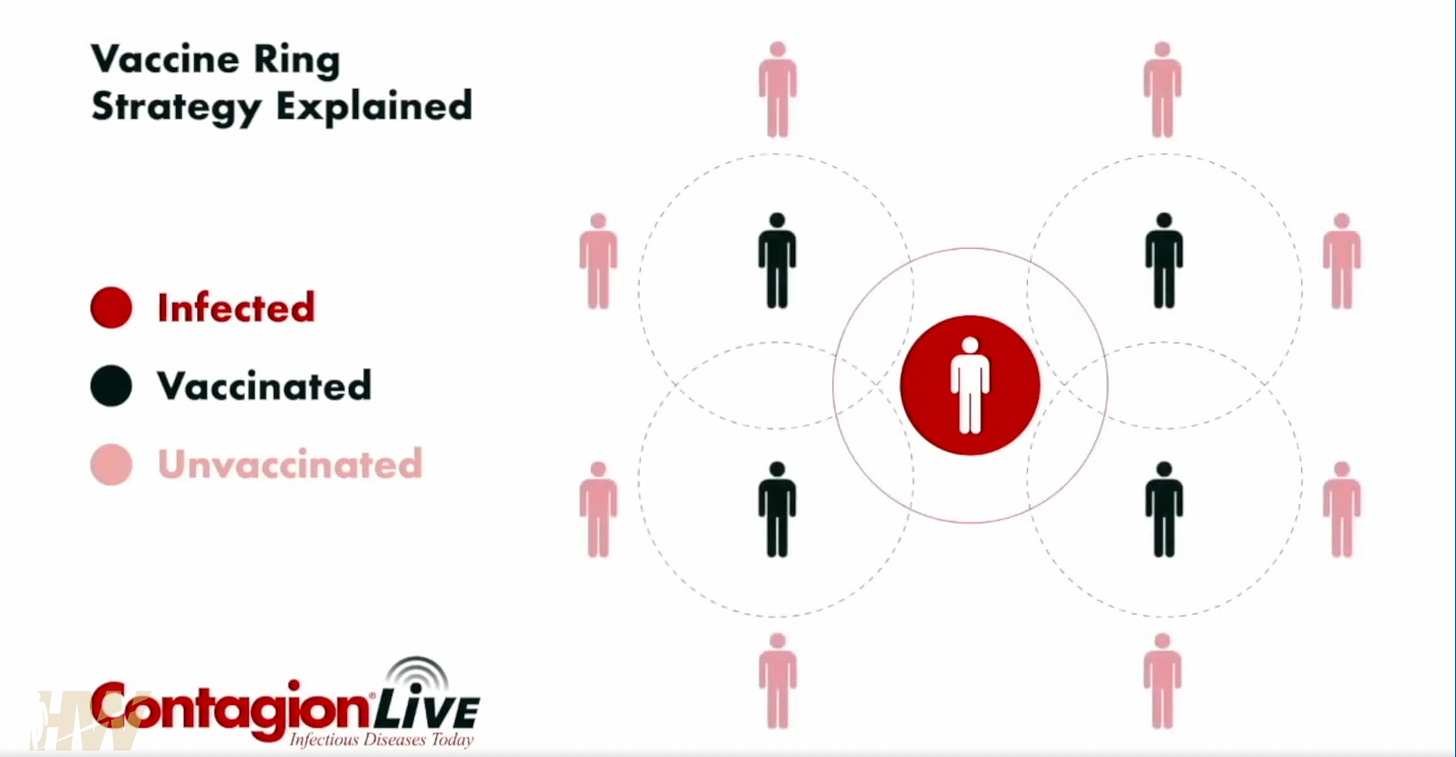
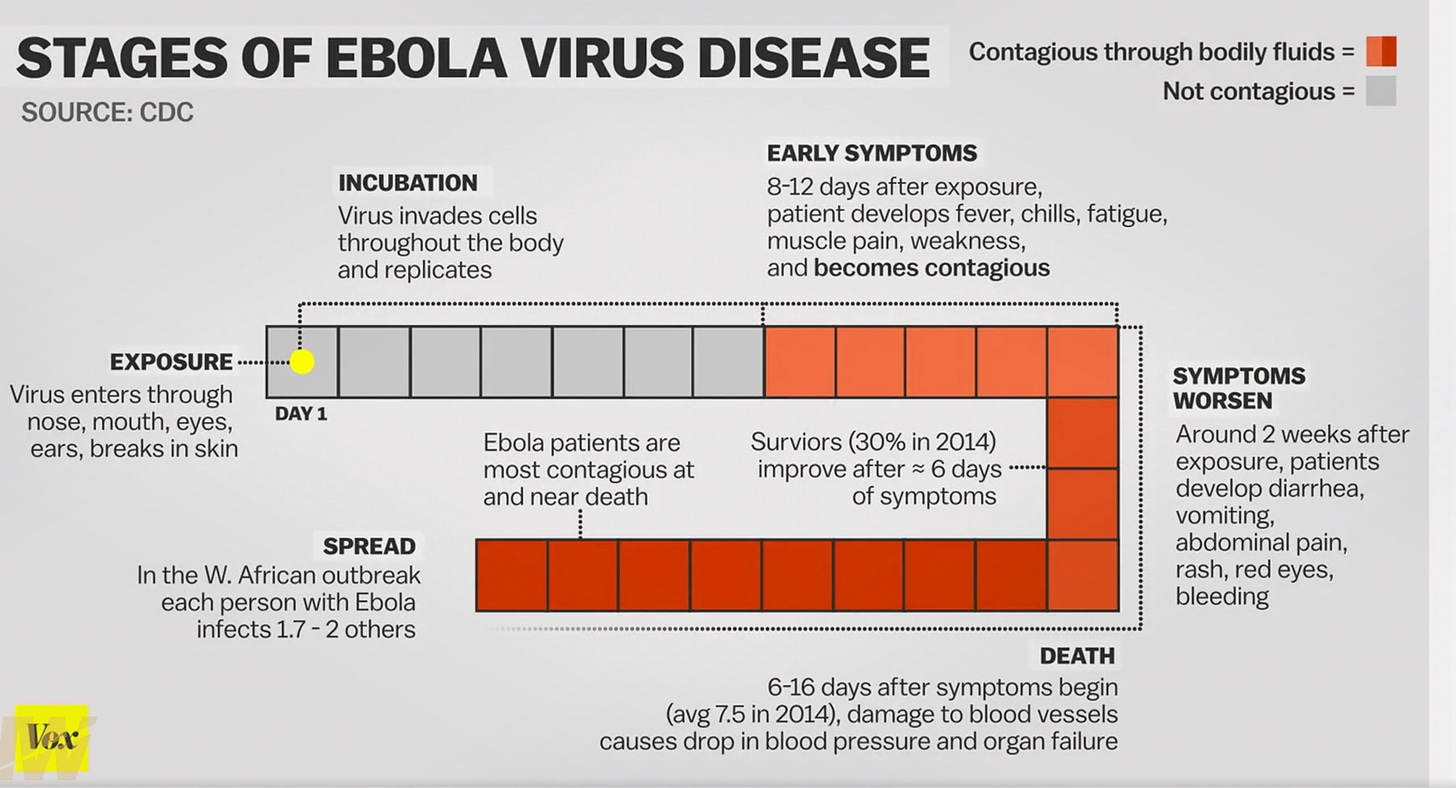
Apologies if I miss some nuance, but this is the layperson interpretation of what went wrong based on GVB’s testimony. The subjects in the trial were “ring vaccinated” after being exposed to the virus by an “index person”. The disease has a roughly ten to twelve day incubation period.
The vaccine candidate itself, rVSV-ZEBOV is highly inflammatory, and thus some of the infected and test subjects were treated to a cytokine storm as the vaccine piled inflammation onto the inflammatory disease that was already incubating, and thus didn’t make it through the ten day incubation period.
😮
Geert sounded the alarm to the WHO about this issue, and asked for the case fatality rate of the entire testing period, but was told that results were confidential. Essentially, GVB was stonewalled.
Later he was tipped off to the Lancet Study crowing about “100% efficacy” of the rVSV-ZEBOV Ebola vaccine.
Seems like they also unblinded the study at least partially if I read it correctly.
On July 31, 2015, random assignment into immediate and delayed vaccination was discontinued on the recommendation of the DSMB, whose decision took into consideration the interim analysis showing 100% vaccine efficacy18 (although they noted that the prespecified α spending criterion of 0·0027 was not achieved) and the low probability of being able to recruit substantial numbers of additional rings (given the declining number of cases of Ebola virus disease in the country). Thereafter, all identified rings received immediate vaccination. Ring enrolment was concluded on Jan 20, 2016.
Any of this sound familiar?
Like unblinding the Pfizer study because “94% effective” and it would be unethical to let the poor control group not have the benefits of the elixir!
Geert is clearly very frustrated at the way that the trials were conducted, and angry with the science being violated.
He mentions a couple of time, “do their homework”.
Discuss.’














Very instructive. Thank you for clarifying the dismal failure of the Ebola clinical trial. Infuriating that GVB was ignored. I look forward to viewing his video in the morning. (Taking so long to load.)
And great job exposing concerns about Malone with careful documentation.
How do we really know that vaccines haven't been killing people for decades? There is no robust system to track the possibility of this and VAERS ain't it.